- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The emerging need for accurate, efficient, inexpensive, and multiparameter monitoring of water quality has led to interest in the miniaturization of benchtop chromatography systems.
This paper reports a chip-based ion chromatography (chip-IC) system in which the microvalves, sample channel, packed column, and conductivity detector are all integrated on a
polymethylmethacrylate (PMMA) chip. A laser-based bonding technique was developed to guarantee simultaneous robust sealing between the homogeneous and heterogeneous interfaces. A
five-electrode-based conductivity detector was presented to improve the sensitivity for nonsuppressed anion detection. Common anions (F−, Cl−, NO3−, and SO42−) were separated in less than 8
min, and a detection limit (LOD) of 0.6 mg L−1 was achieved for SO42−. Tap water was also analyzed using the proposed chip-IC system, and the relative deviations of the quantified
concentration were less than 10% when compared with that a commercial IC system. SIMILAR CONTENT BEING VIEWED BY OTHERS A BATCH MICROFABRICATION OF A SELF-CLEANING, ULTRADURABLE
ELECTROCHEMICAL SENSOR EMPLOYING A BDD FILM FOR THE ONLINE MONITORING OF FREE CHLORINE IN TAP WATER Article Open access 08 April 2022 ON-CHIP-BASED ELECTROCHEMICAL BIOSENSOR FOR THE
SENSITIVE AND LABEL-FREE DETECTION OF _CRYPTOSPORIDIUM_ Article Open access 28 April 2022 DEVELOPMENT OF A ONE-STEP ANALYSIS METHOD FOR SEVERAL AMINO ACIDS USING A MICROFLUIDIC PAPER-BASED
ANALYTICAL DEVICE Article Open access 02 March 2022 INTRODUCTION Safe drinking water is a critical resource for human health1,2. Among a variety of contaminants, some chemical species
present an ongoing concern3. For example, an epidemiological study has shown that long-term exposure to fluoride and nitrate in drinking water can lead to chronic diseases4. In addition,
chloride and sulfate may cause certain aesthetic effects. Therefore, the allowed concentrations of these species in drinking water have been regulated worldwide (Table 1). It is of great
health significance for end-users to measure the concerned ions accurately, inexpensively, and conveniently. Although the foregoing anions can be measured on-site by using simple commercial
test kits based on color reactions on specific strips (Table S1 and Fig. S1), only qualitative results can be obtained, and only a single element can be detected in one test. In contrast,
anions can be quantitatively and simultaneously determined in a laboratory by ion chromatography (IC)5, which is considered the standard method for detecting common cations and anions in
water samples. Unfortunately, current chromatography systems are too bulky for use in field tests6,7. In past decades, advancements in lab-on-a-chip technology have successfully addressed
this issue for both chip-based gas chromatography (chip-GC)8,9,10,11 and chip-based liquid chromatography (chip-LC)12,13,14,15 but relatively less so for chip-IC. Kidd et al.16 packed a
silicon microchannel with anion resin to test common anions by incorporating an external syringe pump, injector, and contactless conductivity detector (C4D). Tanaka et al.17 presented a
polymethylmethacrylate (PMMA) chip packed with anion exchangers to detect glycated hemoglobin (HbA1c). However, the chip was also operated by a commercial syringe pump, valve, and
electrochemical detector. Previously, our group also reported a packed PMMA IC chip for the detection of HbA1c18. Ultraviolet–visible spectroscopy (UV/Vis) detection was performed through a
fiber-coupled microspectrometer. Despite these advances, developing a compact anion-oriented chip-IC is still a challenge. On the one hand, the current chip-IC mainly relies on discrete
commercial parts, and the level of integration needs to be further improved. On the other hand, a lightweight but sensitive on-chip detector for anions should also be developed. Basically,
the primary parts of a chip-IC system comprise a micropump, injector, column, and detector. Considering that band broadening only occurs at the flow path between the sample injection
channel, column, and detection cell12, the integration of a micropump is nonessential, and a traditional LC pump or syringe pump can still be used for chip-IC. Nevertheless, an integrated
micropump can dramatically reduce the size and weight of the whole system19. The column is the most important part of an IC system. Though on-chip columns have been achieved in
open-tubular20,21, monolithic22,23, and pillar array24,25 forms, it is straightforward to pack the channel with commercially available resins, and better reproducibility can be guaranteed
because no surface modification of the particulates is needed26. To keep beads in the channels, frit structures with feature sizes smaller than the resin’s diameter are usually needed.
However, commonly used frits, such as weirs27, step-shaped channels28, and parallel small channels29, follow a similar multistep lithography and etching process, and interfacial bonding is
also severely challenged by the high-pressure resistance of the packed bed. Therefore, more attention should be given to both the chip material and corresponding fabrication. For example,
the commonly used polydimethylsiloxane (PDMS) in microfluidics is limited in chip-LC because PDMS is gas permeable and the bonding strength is relatively poor30. Sample injection on-chip has
been mainly achieved by using T-shaped31,32 or cross-shaped27,33 channels. However, external valves are needed for fluid on/off control at the sample inlet and outlet. In contrast,
integrated microvalves are urgently desired34, but their implementation is restricted by the high working pressure35 or until the poor bonding strength of the heterogeneous interface between
the substrate and valve membrane is improved. Ultrasensitive detectors, such as mass spectrometry (MS)22,28,29,36 and laser-induced fluorescence (LIF)20,21,23, have been widely used in
chip-LC to measure biomolecules, organic compounds, and pharmaceuticals. However, conductivity detection (CD) is the first choice for IC due to its universality to ionic samples37. In
macro-IC systems, a suppressor is commonly used to neutralize the high background conductivity of the eluent before CD to increase the detection sensitivity. Although a suppressor on-chip
system has recently been reported38, the integration of suppressors in a chip-IC system is much more complicated; therefore, nonsuppressed CD, which uses a low-conductivity eluent for direct
CD after separation, is more suitable for chip-IC. In terms of the detector cell, electrodes can be either in direct contact or not with the eluate. Although the contact mode can provide
higher sensitivity than the contactless model39, the electrode reaction may lead to large baseline noise and thus compromise the signal-to-noise ratio (S/N) and sensitivity. Therefore, a
contact CD with steady and reduced baseline noise is helpful to increase the sensitivity in nonsuppressed chip-IC. Herein, we developed a compact chip-IC system for the detection of anions
in drinking water. A channel network with various cavities was micromilled in one step on a PMMA substrate. Commercial sintered frits and valve membrane disks were embedded into the
corresponding cavities for integration. Both homogeneous and heterogeneous interfaces were robustly sealed by laser bonding to resist the high working pressure. A conductivity detector with
a five-electrode configuration was integrated to improve the S/N in the nonsuppressed mode. Separation of both anion standards and tap water was demonstrated by using the proposed chip-IC
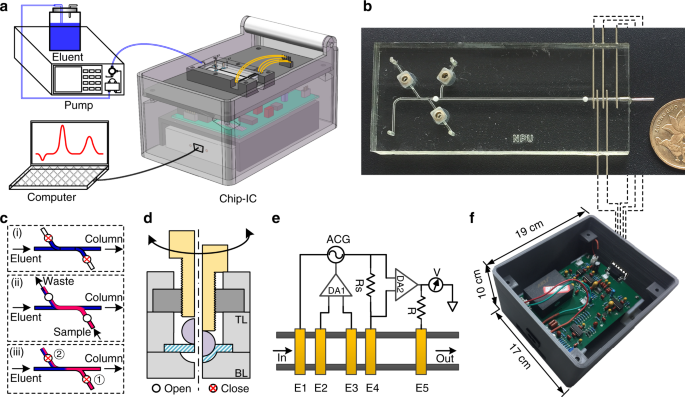
system. RESULTS AND DISCUSSION CHIP-IC SYSTEM Figure 1a presents an overview of the experimental setup. The chip-IC system consists of an IC chip as well as necessary electronics including a
data processing circuit and a data acquisition (DAQ) unit, and it is arranged in a 3D-printed box that is 17 × 19 × 10 cm3 in size and less than 2 kg in weight. A conventional pump is
connected to the chip through PEEK fitting (Agilent, Beijing, China) for eluent delivery. The chromatographic signal is read from the DAQ and recorded on a computer. For chromatographic
separation, the IC chip (Fig. 1b) uses the sample injection procedure shown in Fig. 1c. Briefly, the packed channel is first equilibrated using the eluent to obtain a steady baseline. The
two valves are then opened, and the sample is injected from the inlet. After injection, the two valves are closed sequentially, and the sample plug will be drawn into the packed channel for
separation. All valves are currently driven by screw40,41 (Fig. 1d); however, micromotors or solenoid valves can also be incorporated for automated control40. To obtain an exact on/off
operation under high pressure, a tiny ball is adopted to force the membrane to be in close contact with the hemispheric valve chamber. Figure 1e shows the principle of the five-electrode CD
system. The five electrodes include the two reference electrodes (E1 and E4), two detection electrodes (E2 and E3), and an electrical ground electrode (E5). A sinusoidal current is applied
to E1 and E4 (denoted as I14) and is controlled by a variable AC generator (ACG). E2 and E3 are inputs to a differential amplifier (DA1). When the conductance between E2 and E3 (G23)
changes, the output of DA1 will force the ACG to vary I14 to maintain a constant potential drop between E2 and E3 (U23). In response, the potential drop on the sampling resistor (URs) will
also vary and be further converted by another differential amplifier (DA2) and finally detected by voltmeter V. E5 reduces the capacitance leakage to the ground. Because there is only a very
low current at E2 and E3, common problems in contact mode, such as Faradaic impedance, are largely eliminated. Therefore, the 5e detector will give a more stable baseline and accurate
measurement of the solution conductance, which makes it very suitable for nonsuppressed IC detection42. A developed PCB for signal processing is presented in Fig. 1f. CHIP FABRICATION The
device is fabricated by combining micromilling and laser bonding techniques. The process (Fig. 2a) mainly involves three steps: (1) single-layered chips with various channel geometries are
micromilled43; (2) small chips are cleaned, and a pair of pieces as well as frits, valve membranes, and electrodes are assembled together; and (3) the assembled block is laser-bonded (Fig.
S2). From the infrared view (Fluke Ti400, Beijing, China) of laser bonding (Fig. 2b), only a small local zone of PMMA is heated to high temperature and melts (Fig. 2c). The PMMA melts can
slightly flow into any interfacial gaps, and after resolidification, effective sealing is achieved on both the homogeneous PMMA interface and heterogeneous interfaces, e.g., PMMA/frit,
PMMA/valve membrane, and PMMA/electrode (Fig. 2d, e). The normal stress of bonded chips was measured using a tensile tester (SFMIT, Suzhou, China). Dumbbell-shaped specimens with a 1.5 × 2
mm2 cross-section were tested at a pull rate of 1.0 mm min−1 (Fig. S3). Most (6 of 8) of the specimens were fractured out of the bonded interface, and the average normal strength was
calculated to be 74.5 MPa, with a relative standard deviation (RSD) of 0.64% (_n_ = 8). This value is much larger than that reported by Hsu et al.44 (17.6 MPa) and Tran et al.45 (6.17 MPa).
Sealing between heterogeneous interfaces was tested by using the burst pressure method (Fig. S4). A burst pressure of 10.6 MPa was recorded with leakage occurring at the world-to-chip
connection rather than the chip interface. This value is also much higher than that reported by Hsu et al.44 (0.69 MPa) and is sufficient for column packing and chromatographic separation.
COLUMN PACKING QUALITY The chip was packed using a slurry method (Fig. S5). The dimensionless flow resistance _ϕ_ gives a comparative measure of the packing quality of a column, which is
defined as46 $$\frac{\phi }{\varepsilon } = \frac{{d_{\mathrm{p}}^2A_{\mathrm{c}}}}{{\eta L_{\mathrm{c}}}}\frac{{\Delta p}}{F}$$ (1) where _ε_ is the porosity of the column (typically
~0.65), ∆_p_ is the pressure drop of the column, _d_p is the bead diameter, _A_c is the column cross-sectional area, _L_c is the column length, and _η_ and _F_ are the viscosity and flow
rate of the mobile phase, respectively. Generally, _ϕ_ is 500–1000 for packed columns, and a lower value usually indicates poor packing with voids or cracks. Figure 3 shows the pressure drop
at varying flow rates of DI water, where _ϕ_ is calculated to be 650. This value is well within the typical range of 500–1000, meaning that a favorable packed quality was achieved. In
addition, according to the _ϕ_ value of 355 with assumed _ε_ = 0.8 obtained in a parylene channel packed with the same beads used here46, _ϕ_ is calculated to be 289 with _ε_ = 0.65. The
higher _ϕ_ values here also mean that better packing quality was achieved by following the current column design and packing technique. DETECTION OF ANION STANDARDS A set of SO42− standard
solutions with concentrations of {5, 10, 25, 50, and 100 mg L−1} were tested at a flow rate of 25 μL min−1, with each concentration tested three times. As shown in Fig. 4, good linearity
(_R_2 = 0.996) was observed in the range of 5–100 mg L−1 for the 5e detector. The RSDs of the retention time _t_R and peak height _H_ are 0.2–0.5% and 0.6–2.4%, respectively. Based on the
observed S/N at 5 mg L−1, the S/N = 3 LOD is calculated to be 0.6 mg L−1. Two standard mixtures, i.e., (#1, 10–20–20) containing 10 mg L−1 Cl−, 20 mg L−1 NO3−, and 20 mg L−1 SO42− and (#2,
50–10–30–20) containing 50 mg L−1 F−, 10 mg L−1 Cl−, 30 mg L−1 NO3−, and 20 mg L−1 SO42−, were tested using the packed chip at a flow rate of 25 μL min−1. As shown in Fig. 5a, F− and Cl−
overlap under the current chip and conditions. This overlap may be due to the large water dip in nonsuppressed IC, which has been confirmed in nonsuppressed capillary ICs47. However, the
overlapping peaks of F− and Cl− can be discriminated by postprocessing in software such as OriginPro (Fig. S6). On the other hand, it is very promising to separate F− and Cl− directly by
using more advanced and smaller resins and optimizing the buffer condition. The three-anion mixture (#1, 10–20–20) was tested at varying flow rates. Figure 5b shows that the smaller flow
rate achieves better resolution, although the analysis time is longer. At a flow rate of 25 μL min−1, all three ions can be detected within 8 min. The separation efficiency, namely, the
number of theoretical plates, is defined as _N_ = 5.54(_t_R/_W_h/2)2, where _t_R is the retention time and _W_h/2 is the full width at half maximum (FWHM). As the flow rate varies from 25 μL
min−1 to 10 μL min−1, the _N_ values (plates m−1) for Cl−, NO3−, and SO42− are 3800–7100, 7100–11,200, and 6100–9600, respectively (Table S3). Compared with nonsuppressed CapIC47, chip-IC
showed better efficiency in the separation of relatively strongly retained NO3− (reported _N_ = 5400); nevertheless, the _N_ value of Cl− in chip-IC is only half that reported (_N_ = 15,800)
in CapIC. The large difference in relatively weakly retained species may also be attributed to the large water dip in the nonsuppressed IC. DETECTION OF TAP WATER Tap water was successfully
analyzed using the chip-IC system at room temperature. A water sample was collected directly from the tap, filtered, and then injected for separation. Separation tests were performed at 7
am and 7 pm. Figure 6 and Table 2 show the obtained chromatograms and corresponding concentrations of anions, respectively. Water samples were also tested on a benchtop IC system (IC-8286,
Luhai Photoelectric, Qingdao, China), which confirmed that only F−, Cl−, NO3−, and SO42− were determined. Therefore, no other overlap occurs on the chromatograph by chip-IC except for F− and
Cl−. As mentioned, the overlapping F− and Cl− peaks can be post-treated for peak discrimination. However, the concentration of F− is so small that the postprocessing would result in a large
relative error. To assess the true detectability of the chip-IC system, only NO3− and SO42− data by chip-IC are quantified and presented in Table 2, which reveals that the relative
deviations of concentrations of these anions obtained by chip-IC are less than 10% when compared with the commercial IC-8286 system. MATERIALS AND METHODS REAGENTS Isopropanol, methanol,
_p_-hydroxybenzoic acid (_p_-HBA), sodium chloride, sodium hydroxide, anion stock solutions (1000 mg L−1) of fluoride (F−), chloride (Cl−), nitrate (NO3−), and sulfate (SO42−), and two
standard mixtures—one containing 10 mg L−1 Cl−, 20 mg L−1 NO3−, and 20 mg L−1 SO42− (#1, 10–20–20), and the other containing 50 mg L−1 F−, 10 mg L−1 Cl−, 30 mg L−1 NO3−, and 20 mg L−1 SO42−
(#2, 50–10–30–20)—were all acquired from Sigma–Aldrich (Shanghai, China) at reagent grade. Single-anion calibration standards were prepared by diluting the stock solution with deionized
water to the desired concentration. The eluent was prepared in 4.0 mM _p_-HBA solution containing 2.5% methanol, with the pH adjusted to 8.5 by 0.1 M NaOH. All solutions were filtered using
a 0.2 μm filter before use. CHIP DESIGN AND FABRICATION The device was designed using SolidWorks (Dassault Systemes, Waltham, MA, USA). The whole chip consists of two layers: the bottom
layer (BL) containing the microchannel network including a double-T sample injection junction, valve chamber, frit cavities, separation column, and detection cell and the top layer (TL)
containing the valve membrane cavities, electrode cavities and through vias for valve control and tube connection. The cross-sectional size of the channel is 500 × 500 μm2. A 0.5 µL sample
plug is defined by a double-T-shaped side channel at a distance of 2 mm. The separation channel is 40 mm long. Two frit cavities are set at each side of the separation channel, and the depth
and diameter of each frit cavity are 0.5 mm and 1.4 mm, respectively. The diameter of commercially available ultrahigh-molecular weight polyethylene (UHMW-PE) frits (Biocomma, Shenzhen,
China) is also 1.4 mm. Another side channel is set to introduce the slurry for chip packing. Every side channel has a membrane microvalve integrated for fluid on/off manipulation. The
diameter and depth of the spherical valve chamber are 0.5 mm and 0.6 mm, respectively. The depth and diameter of valve membrane cavities are 4 mm and 0.5 mm, respectively; 4-mm-diameter
disks are cut from a 500-µm-thick highly elastic silicone sheet (Sanhe 3A Rubber & Plastic Co., Hebei, China) to function as valve membranes. The diameters of the through vias for valve
control and tube connection are 1 mm and 3 mm, respectively. The total chip size is 30 × 70 mm2. Additionally, 3-mm-thick PMMA sheets (Evonik Röhm, Darmstadt, Germany) are micromilled to
create channels and then cut into pieces using a CNC machine (Jingdiao, Beijing, China). Small pieces are ultrasonically cleaned in isopropanol for 2 min, rinsed in DI water, dried with
nitrogen, and then laser-bonded at room temperature. Briefly, the Clearweld™ absorber (Crysta-Lyn, Binghamton, NY, USA) is first manually coated on the top surface of the BL. Then, the frits
and membrane disks are inserted into the frit cavities and membrane cavities. Pt wires 0.5 mm in diameter are inserted into the electrode channels. Then, a pair of chips are stacked,
clamped using a homemade fixture, and laser-bonded. The focused spot is ~400 μm, and the optimal laser power, scanning velocity and pitch are 10 W, 8 mm s−1, and 0.4 mm, respectively. COLUMN
PACKING An approximately 80 mg mL−1 slurry of 7 µm PRP-X110 anion exchanger (Hamilton Reno, NV, USA) is prepared in a 2 M NaCl solution and ultrasonicated for 1 min. A PEEK tube (0.5 mm
i.d. and 10 cm long) filled with slurry is connected between the packing inlet and an HPLC pump (Hanbon Sci. & Tech., Huai’an, China). The channel is packed at a flow rate of 200 μL
min−1, which is ten times larger than that for separation. The frit has an average pore size of 2 μm and, thus, can keep the beads within the channel. The maximum pumping pressure is set to
10 MPa, at which point the pump will turn off automatically, the packing valve will close, and the packed channel will depressurize spontaneously. CONCLUSIONS In this paper, a
high-pressure-compatible ion chromatography chip was fabricated by using micromilling and laser-based bonding methods. Owing to the melting–resolidification transition, high bonding strength
has been achieved. Using the integrated five-electrode conductivity detector, standard anion solutions and tap water were successfully analyzed. Although cations have not yet been tested,
theoretically, better results can be obtained using the same system. With further improvements in micropump integration and automated valve control, we believe that a fully portable chip-IC
device would be an alternative tool for field environmental testing, especially for high-throughput detection in water. REFERENCES * Landrigan, P. J. et al. The Lancet Commission on
pollution and health. _Lancet_391, 462–512 (2018). Article Google Scholar * Allaire, M., Wu, H. & Lall, U. National trends in drinking water quality violations. _Proc. Natl Acad. Sci.
USA_115, 2078–2083 (2018). Article Google Scholar * Levallois, P. & Villanueva, C. Drinking water quality and human health: an editorial. _Int. J. Environ. Res. Public Health_16(4pp),
631 (2019). Article Google Scholar * Villanueva, C. M. et al. Assessing exposure and health consequences of chemicals in drinking water: current state of knowledge and research needs.
_Environ. Health Perspect._122, 213–221 (2014). Article Google Scholar * Michalski, R. Ion chromatography applications in wastewater analysis. _Separations_5(12pp), 16 (2018). Article
Google Scholar * Zulkifli, S. N., Rahim, H. A. & Lau, W.-J. Detection of contaminants in water supply: a review on state-of-the-art monitoring technologies and their applications.
_Sens. Actuators B Chem._255, 2657–2689 (2018). Article Google Scholar * Jang, A., Zou, Z., Lee, K. K., Ahn, C. H. & Bishop, P. L. State-of-the-art lab chip sensors for environmental
water monitoring. _Meas. Sci. Technol._22(18pp), 032001 (2011). Article Google Scholar * Wang, J. et al. Compact prototype microfabricated gas chromatographic analyzer for autonomous
determinations of VOC mixtures at typical workplace concentrations. _Microsyst. Nanoeng._4(10pp), 17101 (2018). Article Google Scholar * Zhou, M.-D. et al. Chopper-modulated gas
chromatography electroantennography enabled using high-temperature MEMS flow control device. _Microsyst. Nanoeng._3(10pp), 17062 (2017). Article Google Scholar * Qin, Y. &
Gianchandani, Y. B. A fully electronic microfabricated gas chromatograph with complementary capacitive detectors for indoor pollutants. _Microsyst. Nanoeng._2(11pp), 15049 (2016). Article
Google Scholar * Akbar, M., Restaino, M. & Agah, M. Chip-scale gas chromatography: from injection through detection. _Microsyst. Nanoeng._1(8pp), 15039 (2015). Article Google Scholar
* Haghighi, F., Talebpour, Z. & Nezhad, A. S. Towards fully integrated liquid chromatography on a chip: evolution and evaluation. _Trends Anal. Chem._105, 302–337 (2018). Article Google
Scholar * Yuan, X. & Oleschuk, R. D. Advances in microchip liquid chromatography. _Anal. Chem._90, 283–301 (2018). Article Google Scholar * Grinias, J. P. & Kennedy, R. T.
Advances in and prospects of microchip liquid chromatography. _Trends Anal. Chem._81, 110–117 (2016). Article Google Scholar * Desmet, G. & Eeltink, S. Fundamentals for LC
miniaturization. _Anal. Chem._85, 543–556 (2013). Article Google Scholar * Kidd, R. D. et al. Ion Chromatography-on-a-Chip for Water Quality Analysis. In _Proc. 45th Internationaal
Conference on Environmental Systems__(ICES 2015__)_, 141(8pp), (Bellevue, 2015). * Tanaka, T. et al. On-chip type cation-exchange chromatography with ferrocene-labeled anti-hemoglobin
antibody and electrochemical detector for determination of hemoglobin A1c level. _Anal. Chim. Acta_638, 186–190 (2009). Article Google Scholar * Li, D., Li, X. & Chang, H. An
integrated ion chromatography microchip for ultra fast measurement of glycated hemoglobin levels. in _Proc. IEEE MEMS 2018_, pp 1189–1192. (Belfast, 2018). * Li, X., Li, D., Liu, X. &
Chang, H. Ultra-monodisperse droplet formation using PMMA microchannels integrated with low-pulsation electrolysis micropumps. _Sens. Actuators B Chem._229, 466–475 (2016). Article Google
Scholar * Smirnova, A., Shimizu, H., Pihosh, Y., Mawatari, K. & Kitamori, T. On-chip step-mixing in a T-nanomixer for liquid chromatography in extended-nanochannels. _Anal. Chem._88,
10059–10064 (2016). Article Google Scholar * Fuentes, H. V. & Woolley, A. T. Electrically actuated, pressure-driven liquid chromatography separations in microfabricated devices. _Lab
Chip_7, 1524–1531 (2007). Article Google Scholar * Dietze, C., Hackl, C., Gerhardt, R., Seim, S. & Belder, D. Chip-based electrochromatography coupled to ESI-MS detection.
_Electrophoresis_37, 1345–1352 (2016). Article Google Scholar * Ladner, Y. et al. New ‘one-step’ method for the simultaneous synthesis and anchoring of organic monolith inside COC
microchip channels. _Lab Chip_12, 1680–1685 (2012). Article Google Scholar * Isokawa, M. et al. Liquid chromatography chip with low-dispersion and low-pressure-drop turn structure
utilizing a distribution-controlled pillar array. _Anal. Chem._88, 6485–6491 (2016). Article Google Scholar * Callewaert, M. et al. Integration of uniform porous shell layers in very long
pillar array columns using electrochemical anodization for liquid chromatography. _Analyst_139, 618–625 (2014). Article Google Scholar * Kutter, J. P. Liquid phase chromatography on
microchips. _J. Chromatogr. A_1221, 72–82 (2012). Article Google Scholar * Thurmann, S., Dittmar, A. & Belder, D. A low pressure on-chip injection strategy for high-performance
chip-based chromatography. _J. Chromatogr. A_1340, 59–67 (2014). Article Google Scholar * Xie, J., Miao, Y., Shih, J., Tai, Y. C. & Lee, T. D. Microfluidic platform for liquid
chromatography-tandem mass spectrometry analyses of complex peptide mixtures. _Anal. Chem._77, 6947–6953 (2005). Article Google Scholar * Lazar, I. M. & Kabulski, J. L. Microfluidic LC
device with orthogonal sample extraction for on-chip MALDI-MS detection. _Lab Chip_13, 2055–2065 (2013). Article Google Scholar * Li, X., Chang, H., Liu, X., Ye, F. & Yuan, W. A 3-D
overbridge-shaped micromixer for fast mixing over a wide range of Reynolds numbers. _J. Microelectromech. Syst._24, 1391–1399 (2015). Article Google Scholar * De Malsche, W. et al.
Pressure-driven reverse-phase liquid chromatography separations in ordered nonporous pillar array columns. _Anal. Chem._79, 5915–5926 (2007). Article Google Scholar * Liu, J. et al.
Polymer microchips integrating solid-phase extraction and high-performance liquid chromatography using reversed-phase polymethacrylate monoliths. _Anal. Chem._81, 2545–2554 (2009). Article
Google Scholar * Schlund, M., Gilbert, S. E., Schnydrig, S. & Renaud, P. Continuous sampling and analysis by on-chip liquid/solid chromatography. _Sens. Actuators B Chem._123, 1133–1141
(2007). Article Google Scholar * Lavrik, N. V., Taylor, L. C. & Sepaniak, M. J. Enclosed pillar arrays integrated on a fluidic platform for on-chip separations and analysis. _Lab
Chip_10, 1086–1094 (2010). Article Google Scholar * Li, Y. et al. Miniaturised electrically actuated high pressure injection valve for portable capillary liquid chromatography.
_Talanta_180, 32–35 (2018). Article Google Scholar * Heiland, J. J. et al. On-chip integration of organic synthesis and HPLC/MS analysis for monitoring stereoselective transformations at
the micro-scale. _Lab Chip_17, 76–81 (2017). Article Google Scholar * Yang, B., Zhang, M., Kanyanee, T., Stamos, B. N. & Dasgupta, P. K. An open tubular ion chromatograph. _Anal.
Chem._86, 11554–11561 (2014). Article Google Scholar * Wouters, S., Bruggink, C., Agroskin, Y., Pohl, C. & Eeltink, S. Microfluidic membrane suppressor module design and evaluation for
capillary ion chromatography. _J. Chromatogr. A_1484, 26–33 (2017). Article Google Scholar * Coltro, W. K. T. et al. Capacitively coupled contactless conductivity detection on
microfluidic systems—ten years of development. _Anal. Methods_4, 25–33 (2012). Article Google Scholar * Elizabeth Hulme, S., Shevkoplyas, S. S. & Whitesides, G. M. Incorporation of
prefabricated screw, pneumatic, and solenoid valves into microfluidic devices. _Lab Chip_9, 79–86 (2009). Article Google Scholar * Chen, C. F., Liu, J., Chang, C. C. & DeVoe, D. L.
High-pressure on-chip mechanical valves for thermoplastic microfluidic devices. _Lab Chip_9, 3511–3516 (2009). Article Google Scholar * Haddad, P. R. & Jackson, P. E. in _Journal of
Chromatography Library Volume 46_, 245–289 (Elsevier, 1990). * Guckenberger, D. J., de Groot, T. E., Wan, A. M. D., Beebe, D. J. & Young, E. W. K. Micromilling: a method for ultra-rapid
prototyping of plastic microfluidic devices. _Lab Chip_15, 2364–2378 (2015). Article Google Scholar * Hsu, Y.-C. & Chen, T.-Y. Applying Taguchi methods for solvent-assisted PMMA
bonding technique for static and dynamic μ-TAS devices. _Biomed. Microdevices_9, 513–522 (2007). Article Google Scholar * Tran, H. H., Wu, W. & Lee, N. Y. Ethanol and UV-assisted
instantaneous bonding of PMMA assemblies and tuning in bonding reversibility. _Sens. Actuators B Chem._181, 955–962 (2013). Article Google Scholar * He, Q., Pang, C., Tai, Y.-C. & Lee,
T. D. Ion liquid chromatography on-a-chip with beads-packed parylene column. In _Proc. IEEE MEMS 2004_, pp 212–215, (Maastricht, 2004). * Huang, W. & Dasgupta, P. K. Electrodialytic
capillary suppressor for open tubular ion chromatography. _Anal. Chem._88, 12021–12027 (2016). Article Google Scholar Download references ACKNOWLEDGEMENTS We acknowledge the financial
support from the Shaanxi Key Research and Development Program (Grant No. 2019ZDLGY02-06 & Grant No. 2016KTCQ01-48), the Fundamental Research Funds for the Central Universities (Grant No.
3102019JC002). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Ministry of Education Key Laboratory of Micro/Nano Systems for Aerospace, School of Mechanical Engineering, Northwestern
Polytechnical University, 710072, Xi’an, P. R. China Xiaoping Li & Honglong Chang Authors * Xiaoping Li View author publications You can also search for this author inPubMed Google
Scholar * Honglong Chang View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS H.C. proposed the idea and supervised the study. X.L. performed
the chip design, fabrication, and the experiments. All authors discussed and approved the manuscript. CORRESPONDING AUTHOR Correspondence to Honglong Chang. ETHICS DECLARATIONS CONFLICT OF
INTEREST The authors declare that they have no conflict of interest. SUPPLEMENTARY INFORMATION SUPPLEMENTAL MATERIAL RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a
Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit
to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are
included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Li, X., Chang, H. Chip-based ion chromatography (chip-IC) with a
sensitive five-electrode conductivity detector for the simultaneous detection of multiple ions in drinking water. _Microsyst Nanoeng_ 6, 66 (2020). https://doi.org/10.1038/s41378-020-0175-x
Download citation * Received: 30 December 2019 * Revised: 25 March 2020 * Accepted: 26 April 2020 * Published: 24 August 2020 * DOI: https://doi.org/10.1038/s41378-020-0175-x SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative



:max_bytes(150000):strip_icc():focal(959x0:961x2)/gfm20_covid19-f2c96b28dd6d4b33a61f8c6a36645671.jpg)


