- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT OBJECTIVE To describe outcomes for infants in the neonatal intensive care unit with septic shock based on the vasopressor administered. METHODS This is a multicenter cohort study of
infants with an episode of septic shock. We evaluated the primary outcomes of mortality and pressor-free days alive in the first week after shock using multivariable logistic and Poisson
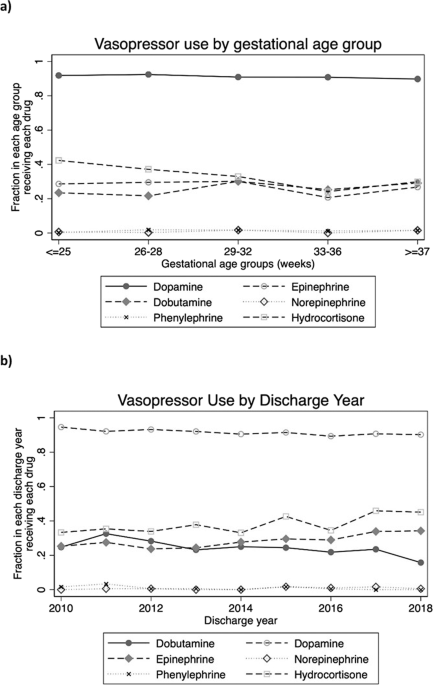
regressions. RESULTS We identified 1592 infants. Mortality was 50%. Dopamine was the most used vasopressor (92% of episodes) and hydrocortisone was co-administered with a vasopressor in 38%
of episodes. Compared to infants treated with dopamine alone, adjusted odds of mortality were significantly higher for those treated with epinephrine alone (aOR 4.7 [95% CI: 2.3–9.2]).
Adjuvant hydrocortisone was associated with significantly lower adjusted odds of mortality (aOR 0.60 [0.42–0.86]) CONCLUSIONS The use of epinephrine as either a solo agent or in combination
therapy was associated with significantly worse outcomes, while adjuvant hydrocortisone was associated with decreased mortality. Access through your institution Buy or subscribe This is a
preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $259.00 per
year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated
during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS EARLY
HYDROCORTISONE VERSES PLACEBO IN NEONATAL SHOCK- A DOUBLE BLIND RANDOMIZED CONTROLLED TRIAL Article 13 February 2025 VASOPRESSIN AS ADJUNCTIVE THERAPY IN PULMONARY HYPERTENSION ASSOCIATED
WITH REFRACTORY SYSTEMIC HYPOTENSION IN TERM NEWBORNS Article 04 July 2024 THE USE OF SUPPLEMENTAL HYDROCORTISONE IN THE MANAGEMENT OF PERSISTENT PULMONARY HYPERTENSION OF THE NEWBORN
Article 15 February 2021 DATA AVAILABILITY The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. CODE
AVAILABILITY The computer code and Stata do-files used to generate the results of the manuscript are available from the corresponding author on reasonable request. REFERENCES * Stoll BJ,
Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2015;314:1039–51. Article CAS
PubMed PubMed Central Google Scholar * Cailes B, Kortsalioudaki C, Buttery J, Pattnayak S, Greenough A, Matthes J, et al. Epidemiology of UK neonatal infections: the neonIN infection
surveillance network. Arch Dis Child Fetal Neonatal Ed. 2018;103:F547–53. Article PubMed Google Scholar * Wynn JL, Wong HR. Pathophysiology and treatment of septic shock in neonates. Clin
Perinatol. 2010;37:439–79. Article PubMed PubMed Central Google Scholar * Burns ML, Stensvold HJ, Risnes K, Guthe HJ, Astrup H, Nordhov SM, et al. Inotropic therapy in newborns, a
population-based national registry study. Pediatr Crit Care Med. 2016;17:948–56. Article PubMed Google Scholar * Tsai M-H, Hsu J-F, Chu S-M, Lien R, Huang H-R, Chiang M-C, et al.
Incidence, clinical characteristics and risk factors for adverse outcome in neonates with late-onset sepsis. Pediatr Infect Dis J. 2014;33:e7–13. Article PubMed Google Scholar * McGovern
M, Giannoni E, Kuester H, Turner MA, van den Hoogen A, Bliss JM, et al. Challenges in developing a consensus definition of neonatal sepsis. Pediatr Res. 2020;88:14–26. Article PubMed
Google Scholar * Verstraete E, Boelens J, De Coen K, Claeys G, Vogelaers D, Vanhaesebrouck P, et al. Healthcare-associated bloodstream infections in a neonatal intensive care unit over a
20-year period (1992-2011): trends in incidence, pathogens, and mortality. Infect Control Hosp Epidemiol. 2014;35:511–8. Article PubMed Google Scholar * Fleiss N, Coggins SA, Lewis AN,
Zeigler A, Cooksey KE, Walker LA, et al. Evaluation of the neonatal sequential organ failure assessment and mortality risk in preterm infants with late-onset infection. JAMA Netw Open.
2021;4:e2036518. Article PubMed PubMed Central Google Scholar * Baske K, Saini SS, Dutta S, Sundaram V. Epinephrine versus dopamine in neonatal septic shock: a double-blind randomized
controlled trial. Eur J Pediatr. 2018;177:1335–42. Article CAS PubMed Google Scholar * Kermorvant-Duchemin E, Laborie S, Rabilloud M, Lapillonne A, Claris O. Outcome and prognostic
factors in neonates with septic shock. Pediatr Crit Care Med. 2008;9:186–91. Article PubMed Google Scholar * Weiss SL, Peters MJ, Alhazzani W, Agus MSD, Flori HR, Inwald DP, et al.
Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 2020;46:10–67. Article CAS
PubMed PubMed Central Google Scholar * Ventura AMC, Shieh HH, Bousso A, Goes PF, Fernandes ICFO, de Souza DC, et al. Double-blind prospective randomized controlled trial of dopamine
versus epinephrine as first-line vasoactive drugs in pediatric septic shock. Crit Care Med. 2015;43:2292–302. Article CAS PubMed Google Scholar * Ramaswamy KN, Singhi S, Jayashree M,
Bansal A, Nallasamy K. Double-blind randomized clinical trial comparing dopamine and epinephrine in pediatric fluid-refractory hypotensive septic shock. Pediatr Crit Care Med.
2016;17:e502–12. Article PubMed Google Scholar * Kharrat A, Jain A. Hemodynamic dysfunction in neonatal sepsis. Pediatr Res. 2022;91:413–24. Article PubMed Google Scholar * Miller LE,
Laughon MM, Clark RH, Zimmerman KO, Hornik CP, Aleem S, et al. Vasoactive medications in extremely low gestational age neonates during the first postnatal week. J Perinatol. 2021;41:2330–6.
Article CAS PubMed PubMed Central Google Scholar * Spitzer AR, Ellsbury DL, Handler D, Clark RH. The Pediatrix BabySteps Data Warehouse and the Pediatrix QualitySteps improvement
project system—tools for “meaningful use” in continuous quality improvement. Clin Perinatol. 2010;37:49–70. Article PubMed Google Scholar * Wynn JL, Polin RA. A neonatal sequential organ
failure assessment score predicts mortality to late-onset sepsis in preterm very low birth weight infants. Pediatr Res. 2020;88:85–90. Article PubMed Google Scholar * Sassano-Higgins S,
Friedlich P, Seri I. A meta-analysis of dopamine use in hypotensive preterm infants: blood pressure and cerebral hemodynamics. J Perinatol. 2011;31:647–55. Article CAS PubMed Google
Scholar * Valverde E, Pellicer A, Madero R, Elorza D, Quero J, Cabañas F. Dopamine versus epinephrine for cardiovascular support in low birth weight infants: analysis of systemic effects
and neonatal clinical outcomes. Pediatrics. 2006;117:e1213–22. Article PubMed Google Scholar * Osborn D, Evans N, Kluckow M. Randomized trial of dobutamine versus dopamine in preterm
infants with low systemic blood flow. J Pediatr. 2002;140:183–91. Article CAS PubMed Google Scholar * Osborn DA, Paradisis M, Evans N. The effect of inotropes on morbidity and mortality
in preterm infants with low systemic or organ blood flow. Cochrane Database Syst Rev. 2007;2007:CD005090. PubMed PubMed Central Google Scholar * Zimmerman K, Gonzalez D, Swamy GK,
Cohen-Wolkowiez M. Pharmacologic studies in vulnerable populations: using the pediatric experience. Semin Perinatol. 2015;39:532–6. Article PubMed PubMed Central Google Scholar * Ibrahim
H, Sinha IP, Subhedar NV. Corticosteroids for treating hypotension in preterm infants. Cochrane Database Syst Rev. 2011;2011:CD003662. PubMed PubMed Central Google Scholar * Crouchley
JL, Smith PB, Cotton CM, Hornik CD, Goldberg RN, Foreman JW, et al. Effects of low-dose dopamine on urine output in normotensive very low birth weight neonates. J Perinatol. 2013;33:619–21.
Article CAS PubMed PubMed Central Google Scholar * Schmatz M, Srinivasan L, Grundmeier RW, Elci OU, Weiss SL, Masino AJ, et al. Surviving sepsis in a referral neonatal intensive care
unit: association between time to antibiotic administration and in-hospital outcomes. J Pediatr. 2020;217:59–65.e1. Article PubMed Google Scholar * Kumar A, Roberts D, Wood KE, Light B,
Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med.
2006;34:1589–96. Article PubMed Google Scholar Download references ACKNOWLEDGEMENTS Erin Campbell, MS, provided editorial review and manuscript submission. Erin Campbell did not receive
compensation for her assistance, apart from her employment at the institution where this research was conducted. Carol Hill, PhD, provided informatics assistance with data procurement from
Pediatrix. The authors would like to thank Duke Pediatric Research Scholars. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pediatrics, Duke University School of Medicine,
Durham, NC, USA Henry P. Foote, Rachel G. Greenberg & Christoph P. Hornik * Department of Economics, Clemson University, Clemson, SC, USA Daniel K. Benjamin * Duke Clinical Research
Institute, Durham, NC, USA Rachel G. Greenberg & Christoph P. Hornik * Pediatrix Medical Group, Inc., Sunrise, FL, USA Reese H. Clark Authors * Henry P. Foote View author publications
You can also search for this author inPubMed Google Scholar * Daniel K. Benjamin View author publications You can also search for this author inPubMed Google Scholar * Rachel G. Greenberg
View author publications You can also search for this author inPubMed Google Scholar * Reese H. Clark View author publications You can also search for this author inPubMed Google Scholar *
Christoph P. Hornik View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS All authors made substantial contributions to the conception or design
of the work, or the acquisition, analysis, or interpretation of data. HPF drafted the work, all authors revised it critically for important intellectual content. All authors approved the
final version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are
appropriately investigated and resolved. CORRESPONDING AUTHOR Correspondence to Christoph P. Hornik. ETHICS DECLARATIONS COMPETING INTERESTS RGG received support from the industry for
research services (https://dcri.org/about-us/conflict-of-interest/). The authors have no other conflicts of interest relevant to this article to disclose. ETHICS APPROVAL AND CONSENT TO
PARTICIPATE We obtained institutional review board approval from the Duke University Health System IRB with a waiver of informed consent for our study, protocol Pro00112607. The study was
performed in accordance with the Declaration of Helsinki. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY MATERIAL RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights
to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the
terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Foote, H.P., Benjamin, D.K., Greenberg, R.G. _et al._ Use of vasopressors
for septic shock in the neonatal intensive care unit. _J Perinatol_ 43, 1274–1280 (2023). https://doi.org/10.1038/s41372-023-01667-8 Download citation * Received: 09 January 2023 * Revised:
21 March 2023 * Accepted: 24 March 2023 * Published: 13 April 2023 * Issue Date: October 2023 * DOI: https://doi.org/10.1038/s41372-023-01667-8 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative







