- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BACKGROUND Characterizing retrospective exposure to toxicants during multiple early-life developmental periods is challenging, yet critical for understanding developmental effects.
OBJECTIVE To characterize early-life metal exposure using deciduous teeth in a community concerned about past exposures. METHODS Naturally shed teeth were collected from 30 children ages
5–13 years who resided in Holliston, Massachusetts since conception. We estimated weekly prenatal and postnatal (up to 1 year of age) exposure to 12 metals by measuring dentine
concentrations using laser ablation-inductively coupled plasma-mass spectrometry. Multivariable linear mixed models were used to explore sociodemographic, dietary, and behavioral correlates
of dentine metal concentrations. RESULTS Temporal trends in dentine levels differed by metal. Source of milk during the first year of life was associated with dentine barium (Ba) levels,
where being fed predominantly breastmilk was associated with 39% (95% CI: –57%, –13%) lower dentine Ba compared to predominantly formula use. Females had higher prenatal and postnatal
dentine Mn and Pb, compared to males (e.g., % difference, postnatal Mn: 122% (17%, 321%); postnatal Pb: 60% (95% CI: –8%, 178%)). SIGNIFICANCE Deciduous teeth provide retrospective
information on dose and timing of early-life metals exposure at high resolution. We demonstrate their utility in a community-based study with known past contamination of drinking water.
IMPACT STATEMENT We conducted a community-initiated pilot study in a community concerned with historical exposure to multiple metals. Using deciduous teeth, a novel noninvasive biomarker, we
characterized early-life exposure to 12 metals in approximately weekly increments during sensitive developmental periods, thus demonstrating the utility of this biomarker in communities
concerned with past exposures. SIMILAR CONTENT BEING VIEWED BY OTHERS QUANTIFIED RETROSPECTIVE BIOMONITORING OF FETAL AND INFANT ELEMENTAL EXPOSURE USING LA-ICP-MS ANALYSIS OF DECIDUOUS
DENTIN IN THREE CONTRASTING HUMAN COHORTS Article 12 February 2024 INFANCY, CHILDHOOD, AND PUBERTY ON THE SILK ROAD REVEALED WITH ISOTOPIC ANALYSIS OF INCREMENTAL DENTINE Article Open access
14 November 2022 LOW LEVELS OF SALIVARY METALS, ORAL MICROBIOME COMPOSITION AND DENTAL DECAY Article Open access 04 September 2020 INTRODUCTION Critical windows of susceptibility, also
referred to as critical windows of exposure, are periods during development in which exposures to specific toxicants have a greater effect on a health outcome compared to the same exposure
during other periods [1]. During development, the brain undergoes unique changes in morphology and connectivity, making gestation and infancy critical periods during which exposure to
toxicants often have adverse effects on neurodevelopment [2, 3]. Furthermore, protective regulatory mechanisms such as the blood–brain barrier are not fully formed, making the fetus and
infant particularly susceptible to overexposure to toxicants [4, 5]. Increasing evidence points to heterogeneity in the health effects of metals depending on timing of exposure [5, 6]. Thus,
it is important to characterize metal exposure during multiple developmental periods, rather than at a single time point. Measuring retrospective exposure, particularly during critical
periods such as in utero and early childhood, presents a challenge in environmental epidemiology studies. While estimates of past exposure, such as historic public drinking water or air
monitoring data, are sometimes available that coincide with the critical windows of interest, these environmental measures have greater error compared to internal biomarkers for measuring
individual-level exposure, given that they are further removed from the target tissue dose [7]. Traditional biomarkers of prenatal and early-life exposure (e.g., maternal or cord blood) are
typically collected less frequently, are limited in the information they provide (i.e., may represent shorter exposure windows), and are subject to exposure misclassification (i.e., do not
reflect direct exposure to the fetus). Collection of other biospecimens (e.g., hair, nails, urine) from infants and young children can be logistically difficult and would be required at
multiple time points after birth, as these biospecimens represent relatively short windows of exposure. The use of deciduous, or naturally shed, baby teeth presents an opportunity to assess
retrospective early-life exposure to toxicants in a highly temporally resolved manner and thus allows for estimation of exposure in a noninvasive sample during relevant and sensitive
developmental windows [8, 9]. Metals accumulate in tooth dentine beginning in the second trimester of pregnancy and tooth mineralization occurs over time, spanning the prenatal and early
childhood periods [8, 9]. At birth, the neonatal line, a histological feature, is formed in the tooth dentine, allowing researchers to characterize metal exposure during the prenatal,
postnatal, and early childhood periods with temporal precision [8, 9]. Furthermore, a single tooth provides high-resolution repeated measures of exposure that increases the amount of data
per individual and avoids the logistical challenges of collecting repeated biospecimens, which is necessary when examining critical windows of susceptibility using other biomarkers.
Retrospective biomarkers that can measure levels of multiple contaminants are particularly useful in settings where it is necessary to quantify internal levels of numerous known prior
exposures. For example, regulated (e.g., Pb, having an Action Level under the Safe Drinking Water Act) and unregulated (e.g., Mn, with only a non-enforceable guideline in drinking water)
metals have been detected in public drinking water supplies in the US and globally at levels that may pose a public health risk [10,11,12]. Increasing evidence links exposure to metals in
drinking water with decrements in neurobehavioral outcomes in children [11] but the levels of unregulated contaminants, like Mn, in drinking water that are considered safe, particularly for
vulnerable subpopulations like children, are unknown [11, 13]. Some limitations of previous epidemiological studies of metals in drinking water include the lack of a consistent biomarker
across studies and the inability to measure past exposure during the most relevant exposure windows [11]. Thus, the application of the tooth biomarker may be particularly useful in the
context of known past contamination and in community-based studies, in which sample sizes are often small, there may be multiple contaminants of concern, and it is rarely possible to
reconstruct personal exposures accurately [14]. We conducted a community-initiated pilot study in the suburban town of Holliston, Massachusetts (MA), USA. In Holliston, the community relies
on two local aquifers for nearly 100% of their drinking water, which are susceptible to landfill and industrial contamination and contain naturally elevated levels of Mn and other metals, as
evidenced by episodic discolored residential tap water that has occurred for years [15]. This study was conducted in response to residents’ concerns about early-life exposure to metals and
their children’s health. The study aims to assess children’s exposure during critical periods of development, with regular bidirectional communication with community members. By estimating
exposure to multiple metals during sensitive life stages (in utero through 1 year of life) using deciduous teeth as a novel, high-resolution biomarker of retrospective exposure, we conducted
an exploratory analysis to examine demographic, behavioral, and water consumption factors as correlates of dentine metal concentrations. MATERIALS AND METHODS STUDY AREA AND RECRUITMENT
Participants were part of the Assessing Children’s Environmental Exposures (ACHIEVE) study, a community-initiated pilot research study in Holliston, MA, USA. Mother–child pairs were
recruited through local newspapers, posting of brochures on town bulletins, community Facebook pages, and word of mouth. Eligibility criteria included: being a resident of Holliston, MA, at
time of enrollment; having lived in Holliston during pregnancy with participating child; having a child between 5 and 13 years old at time of enrollment who had lost or were losing teeth;
and being willing to donate their child’s shed tooth. If multiple children in a household were eligible, one child per household was included in the study, selected at random. Eligible
participants were informed about the study and written consent was obtained from mothers prior to participation. Of 35 mother–child pairs who expressed interest in the study, 5 were
ineligible because the child was either outside of the eligible age range (_n_ = 2) or did not have a deciduous tooth to donate (_n_ = 3). A total of 30 mother–child pairs were enrolled
between 2017 and 2018, 29 of whom donated a tooth prior to analysis in 2018. Home visits were conducted to collect deciduous teeth and administer questionnaires to mothers. Information was
obtained via questionnaire on participant sociodemographic characteristics, drinking water consumption patterns, use of filters for drinking water, sources of milk during infancy, and
parent-reported learning and behavioral disorders. The research study protocol and all study materials were approved by the Institutional Review Board at the Boston University School of
Public Health. METALS IN DECIDUOUS TEETH One deciduous tooth was collected per child during the home visit. Molars and incisors that had been shed prior to or during enrollment were
collected and stored at room temperature before being shipped to the Senator Frank R. Lautenberg Health Sciences Laboratory at the Icahn School of Medicine at Mount Sinai for analysis of
metals content. Teeth that had defects or high levels of attrition (i.e., excessive wear and tooth surface loss) (_n_ = 1) were unable to be analyzed and were thus excluded. Analytic methods
for measurement of dentine metal concentrations have been validated and described previously in detail [8, 16, 17]. Using the neonatal line formed at birth, incremental markings on the
teeth akin to growth rings in trees are used to assign temporal sampling points (about every 7–10 days). Using laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS), signal
intensities (i.e., values) of the following 12 metals were measured: Mn, Pb, barium (Ba), chromium (Cr), cobalt (Co), copper (Cu), lithium (Li), magnesium (Mg), molybdenum (Mo), strontium
(Sr), tin (Sn), and zinc (Zn). The panel of metals represents the standard panel that can be reliably measured by LA-ICP-MS in the tooth matrix. Calcium (Ca) was also measured to account for
individual mineral density variation within and between participants and metal values were normalized to Ca ion values (e.g., 55Mn:43Ca ratio for manganese). The metal value in the gas
blank was subtracted from the metal values measured in each tooth prior to normalizing to Ca. Tooth metal values that are less than levels measured in the blank (and thus are negative
values) represent levels below the limit of detection (LOD). Detection frequency was above 97% for all metals except Co and Mo (78% and 89%, respectively) (Supplementary Table 1). Given the
relatively low percentage of values below the LOD [18], samples below the LOD were set to the metal’s lowest measured level divided by square root of 2 (Supplementary Table 1). To allow for
a comparison of dentine metal levels between this study and other populations, an external standard correction was used to account for differences between laboratories and analytical
methods. The certified reference material NIST 610 was applied to the dentine metals data for external correction. Laboratory technicians were blinded to participant information. POTENTIAL
CORRELATES OF DENTINE METAL CONCENTRATIONS The following demographic, dietary, and behavioral characteristics were considered as potential correlates of dentine metal concentrations: child
sex, birth order, age (continuous, in years), public versus private drinking water source, filter use during pregnancy, tooth type (incisors versus molars), maternal water consumption habits
during pregnancy, maternal anemia during pregnancy, infant formula use, and breastfeeding. For water consumption habits during pregnancy, mothers reported their frequency of use of filtered
water for food preparation, filtered water for making coffee/tea, and bottled water. Mothers reported duration of breastfeeding (<3 months, 3–6 months, 6–9 months, 9 months–1 year, >1
year), ever use of formula in infant’s first year, infant’s age at first use of formula, and percentage of milk in infant’s first year that was formula (<10%, 10–<50%, 50%,
>50–<100%, 100%). Information on breastfeeding duration and percentage of milk that was formula in infant’s first year were combined to estimate the predominant source of milk in
infant’s first year (breastmilk, formula, or mix). Given the small sample size for some of these groups, the following correlates are presented in supplementary tables only: well type,
filtered water use for food preparation, filtered water use for coffee/tea preparation, bottled water use, and breastfeeding (ever). For temporality reasons, source of milk was evaluated as
a correlate of postnatal levels of dentine metals only. STATISTICAL ANALYSES Summary statistics were calculated, and distributions were examined for all variables (Tables 1 and 2). Dentine
metal levels, measured as a ratio of metal to calcium (i.e., metal:Ca), represent approximately weekly measurements of exposure for each participant from the second trimester of pregnancy
through 1 year of age. For summary statistics only, we averaged the fine-resolution tooth metal measurements within each subject for each of two exposure periods: prenatal (second trimester
to birth) and postnatal (birth through 1 year of age) (Supplementary Table 2). To investigate correlates of dentine metal levels, we used a multivariable linear mixed model with the
fine-resolution dentine metal values (approximately weekly measurements) as the dependent variable. We examined correlates separately for each developmental period, given the potential for
distinct associations in these two different windows. Thus, two sets of models were fit: one for the prenatal period and one for the postnatal period (i.e., weekly measurements from the
second trimester until birth as the dependent variable in prenatal models; from birth to 1 year of age in postnatal models). Dentine metal values were right skewed; we log-transformed metal
values to reduce the influence of extreme values and to meet the normality of residuals assumption for linear regression. A subject-specific random intercept accounted for within-subject
correlation of repeated weekly measures of dentine metal values. All models were adjusted for child sex. Percent difference (%D) in dentine metal value per unit increase in the correlate was
calculated by exponentiating the beta coefficient, subtracting 1, and then multiplying by 100 (i.e., (ebeta − 1) × 100)) (Tables 3–5 and Supplementary Tables 3–14). In a sensitivity
analysis, we computed associations between correlates and tooth levels truncated to postnatal week 20, given the reduced number of sampling points that could be collected from teeth in our
sample after 20 weeks post birth (Supplementary Table 14). All statistical analyses were conducted using R 3.5.2 and SAS 9.4 (SAS Institute Inc.). RESULTS PARTICIPANT CHARACTERISTICS A total
of 28 children with complete questionnaire information and tooth metals data were included in the final analysis. Participating mothers were mostly white (93%) and had lived in the same
home in Holliston, MA, since pregnancy (82%) (Table 1). Of the 26 children with siblings, nearly half were first-born (_n_ = 11; 42%). About one-third of children required special services
at school (_n_ = 9, 32%), nearly all of whom had an individualized education plan (_n_ = 8). Most mothers resided in homes serviced with water from a public drinking water (groundwater)
source during pregnancy (89%) (Table 2). Fifty percent of participants had some type of water filtration system in their homes, including point of use (e.g., sink attachment or pitcher
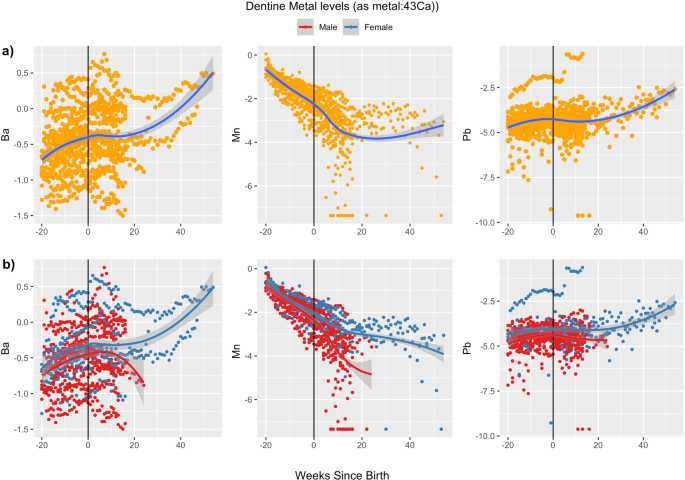
filter) and point of entry (e.g., whole house filter) systems. PRENATAL AND EARLY POSTNATAL VALUES OF DENTINE METALS We measured 12 metals in dentine but focus results on Ba, Mn, and Pb,
given the historical evidence of Mn in Holliston drinking water [15], the known neurotoxicity of Mn and Pb, the interests of community members, and prior findings of tooth Ba as a dietary
marker [19]. The total number of measurements of dentine metals values per participant varied, with a mean (range) of 36 (19–70) measurements. Levels and ranges of dentine Ba remained
similar between the prenatal and postnatal period (Fig. 1). In the prenatal period, median (25th, 75th percentile) dentine Ba (as 138Ba:43Ca) was 0.62 (0.49, 0.71), and in the early
postnatal period, the median was 0.64 (0.53, 0.94) (Supplementary Table 2). Levels of dentine Mn were highest in the early prenatal period (second trimester) and declined steadily until
birth, with a slower decline and increased variability in the postnatal period. In the prenatal period, median (25th, 75th percentile) dentine Mn (as 55Mn:43Ca) was 0.25 (0.19, 0.29), while
in the early postnatal period, the median was 0.06 (0.04, 0.09). Like Ba, ranges of dentine Pb remained similar between prenatal and postnatal periods. In the prenatal period, median (25th,
75th percentile) dentine Pb (as 208Pb:43Ca) was 0.01 (0.01, 0.02), and in the early postnatal period, the median was 0.01 (0.01, 0.02). CORRELATES OF DENTINE METALS VALUES Direction and
magnitude of associations between correlates and dentine metals values varied by metal. Being fed predominantly breastmilk, compared to predominantly infant formula, was associated with 39%
(95% CI: –57%, –13%) lower levels of dentine Ba [_ß_ = –0.5 (95% CI: –0.9, –0.1)]; being fed a mix of breastmilk and formula, compared to predominantly formula, was associated with 14%
(–44%, 32%) lower dentine Ba levels [_ß_ = –0.1 (95% CI: –0.6, 0.3)] (Table 3). Similarly, ever use of formula, compared to never use, was associated with higher dentine Ba (%D: 79%; 95% CI:
27%, 152%). For dentine Mn, child sex was the strongest correlate: female sex was associated with 13% (95% CI: –16%, 52%) higher prenatal and 122% (17%, 321%) higher postnatal tooth Mn
values, compared to males (Table 4). Females, compared to males, also had higher levels of Ba and Pb in both the prenatal [%D Ba: 15% (95% CI: –14%, 55%); %D Pb: 40% (95% CI: –9%, 117%)] and
postnatal [%D Ba: 13% (95% CI: –21%, 60%); %D Pb: 60% (95% CI: –8%, 178%)] periods (Table 5), though estimates were imprecise. Associations between other correlates and dentine Ba, Mn, and
Pb were weaker and less precise (Tables 3–5; Supplementary Tables 3 and 4). Time trends in dentine metals values also appeared to differ by sex for Li and Zn, while values of Co, Cr, Cu, Mo,
Mg, and Sn differed little by sex and were less variable over time (Fig. 2 and Supplementary Table 2). Correlates were not appreciably associated with other metals (Co, Cr, Cu, Li, Mo, Mg,
Sn, Sr, Zn) (Supplementary Tables 5–13). There were no appreciable differences in findings from the truncated data set compared to the full data set; thus, we included all data in main
analyses (Supplementary Table 14). DISCUSSION In this community-initiated pilot study, we characterized retrospective exposure to multiple metals during critical developmental periods using
deciduous teeth as a biomarker of prenatal and postnatal exposure. This effort built off our previous work to address community concerns about historical exposures to neurotoxic metals [15].
These data demonstrate the feasibility of employing naturally shed teeth to understand past exposures in a community with known exposure through drinking water, but where retrospective
exposure information on an individual level was otherwise not obtainable. Temporal trends of dentine metal values differed by metal. Dentine Mn levels decreased from the second trimester
until birth whereas dentine Ba slightly increased and Pb remained more constant throughout the prenatal period and early life. We also found that dentine Ba may reflect dietary practices
related to milk consumption during early life and that dentine levels of both Pb and Mn differ by child sex, with females having higher levels than males, although estimates were imprecise.
The observed decrease in dentine Mn from the second trimester to the first year of life has been reported in other studies and is likely related in part to the physiologic need for Mn as an
essential nutrient to support healthy growth in early development [20,21,22]. The prenatal period is a time of rapid growth and development of the fetus, during which the demand for Mn may
be greatest [21]. This is compatible with previous studies that have reported increases in maternal blood Mn during pregnancy [23,24,25], which supports the notion of a biological role of Mn
during gestation. During gestation, exposure to Mn is more tightly regulated as the placenta may protect the fetus from direct effects of Mn overexposure [26], whereas after birth, the
placenta no longer regulates Mn levels that are transported to the fetus. While development continues, the relative demand for Mn may be lower than in the prenatal period. The observed
increase in variability in the postnatal period may be related to both differential exposures in the postnatal versus prenatal environments, such as direct exposure to Mn in drinking water,
as well as biological changes, such as varied absorption of Mn in the gut in the postnatal period compared to the prenatal period [27, 28]. In this study, Ba levels increased slightly from
the prenatal to postnatal periods. An increase in Ba levels after birth is expected, as transfer of Ba from mother to fetus is restricted by the placenta in the prenatal period [27], but
after birth, increasing Ba levels likely reflect consumption of breastmilk and/or formula [19]. One previous study found that 138Ba:43Ca distributions in teeth accurately reflect the dietary
transition from mother’s milk to other sources during the weaning process and concluded that 138Ba:43Ca ratios in teeth could be used to assess dietary transitions from predominantly
breastmilk to predominantly infant formula or solid food intake [19]. In that study, concentrations of 138Ba:43Ca were higher in individuals who reported formula use compared to
breastfeeding [19], which is consistent with our findings. While there is limited evidence that Ba is neurotoxic, toxicological evidence has shown that Ba acts as a competitive potassium
channel antagonist leading to decreased potassium in blood plasma [29]. Given that many metals have been shown to cause increased intracellular Ca [30, 31], and Ca-gated potassium channels
regulate intracellular Ca levels [32], Ba may play an important role in the joint or interactive effects of multiple metals. The use of deciduous teeth as a biomarker of exposure is a novel
technique that is rapidly evolving; as such, few studies have measured metals with similar fine-scale resolution and with the same analytic technique as in our study. Using externally
corrected values, we were able to compare trends between the ELEMENT cohort, a prospective birth cohort in Mexico City where no specific environmental source of Mn exposure was identified,
and our ACHIEVE study [21]. Dentine Mn levels and trends over time were similar between the two cohorts. Furthermore, few studies have examined correlates of metals in early life [21,
33,34,35,36,37,38], and of those, only a handful have looked at correlates of tooth metals [21, 37, 38], given the novelty of the tooth biomarker. In the Center for the Health Assessment of
Mothers and Children of Salinas (CHAMACOS) cohort in Salinas Valley, CA, authors found that application of Mn-containing fungicides, take-home occupational variables (e.g., storing farm
shoes inside the home), and maternal smoking were associated with higher levels of tooth Mn levels in the prenatal period [37]. In an urban Los Angeles, CA community residing near a battery
smelter, maternal education, child sex, proximity to freeways, breastfeeding practices, smoking, and working in a Pb-exposed occupation were associated with soil and tooth levels of Pb in
early life [38]. In the ELEMENT study, mother’s age at birth was associated with dentine Pb, but not dentine Mn, while child’s sex, maternal education, and maternal report of smoking were
not associated with either Mn or Pb levels [21]. In our data, the levels of Mn and Pb tooth levels varied by child sex and this difference was more prominent in the postnatal period. There
is both epidemiological and toxicological evidence suggesting that females, compared to males, may have increased Mn absorption [28, 39,40,41]. Furthermore, a recent study of US adults
reported significantly higher blood Mn levels in females compared to males [40], suggesting there may be potential differences in Mn absorption or metabolism between females and males. One
hypothesis for the difference between females and males may be related to iron (Fe) status because women are more likely to be iron deficient [28, 42, 43]. Mn and Fe share common absorption
and transport pathways [44, 45] and increased Mn absorption may be due to lower Fe levels among females and/or upregulation of these shared mechanisms of Mn and Fe gastrointestinal uptake
[46, 47]. Whether similar mechanisms are at play among infants to impact dentine Mn levels is unknown. Our pilot study demonstrates the feasibility of obtaining retrospective data on a large
panel of metals to address community concerns about past exposures. The tooth biomarker is particularly useful in settings where past exposures that occurred in early life are of interest
and shed baby teeth are readily available. On the other hand, the tooth biomarker is less useful in scenarios that require immediate exposure information, such as crisis management, and/or
where children are too young to shed teeth. As a pilot study, the sample size is small; therefore, there is limited statistical power to detect associations between correlates and tooth
metals. However, up to 70 measurements of metal values were obtained from each tooth, yielding repeated outcome measurements for each participant, which increases statistical power. Our
study also demonstrates that teeth allow for the direct examination of fetal and infant metal levels, rather than using surrogates of prenatal exposure (e.g., maternal blood) or biomarkers
of current or recent childhood exposure (e.g., hair, toenails, blood). While it is unclear which portion of dentine metal levels may reflect endogenous versus exogenous sources, previous
epidemiologic studies have found correlations between metals in environmental matrices and dentine levels [37, 38, 48]. Furthermore, a study in rats found that tooth Mn levels were strongly
correlated with oral dose of Mn as well as with blood and brain Mn [16], suggesting that teeth capture exogenous oral exposure to Mn. The rate of special services required at school was high
among participants of this pilot study (32.1%) compared to the US national rate (14.0%) [49] and to the rate within Holliston for the 2020–2021 academic year (20.6%) [50]. It is possible
that parents who suspected that their children were exposed to metals in early life and whose children require special services in school were more likely to enroll in this study than
parents of children without special services. However, given that tooth metal values were unknown at the time of enrollment and that we did not estimate associations between metals and use
of special services, selection bias should not impact findings of the current study. We also lack information on potential key covariates including duration of pregnancy (i.e., gestational
age) and other dietary sources. In addition, there is potential for misclassification of this self-reported covariate information, though this misclassification is likely to be
nondifferential with respect to tooth metal levels because participants were unaware of their children’s tooth metal levels. Public health recommendations to decrease exposure to
environmental contaminants are often targeted toward protecting the most vulnerable (i.e., those more likely to be exposed) and susceptible (i.e., those more likely to experience an adverse
effect) populations. Although it is well documented that infants and children are both vulnerable and susceptible to toxic exposures [1, 2, 5], understanding the precise timing of toxic
exposures that lead to greater adverse effects will better inform interventions to decrease exposure during critical developmental windows. In this community-initiated pilot study, deciduous
teeth provided the ability to assess exposure retrospectively and noninvasively to multiple metals with high temporal resolution. Deciduous teeth may serve as a useful tool for communities
that have experienced historic environmental exposures. REFERENCES * Selevan SG, KimmelA CA, Mendola P. Identifying critical windows of exposure for children’s health. Environ Health
Perspect. 2000;108:5. Google Scholar * Meredith RM, Dawitz J, Kramvis I. Sensitive time-windows for susceptibility in neurodevelopmental disorders. Trends Neurosci. 2012;35:335–44. Article
CAS Google Scholar * Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20:327–48. Article Google Scholar * Grandjean DP. Neurobehavioural effects of
developmental toxicity. Lancet Neurol. 2014;13:9. Article Google Scholar * Wright RO. Environment, susceptibility windows, development and child health. Curr Opin Pediatr. 2017;29:211–7.
Article Google Scholar * Bauer JA, Fruh V, Howe CG, White RF, Claus Henn B. Associations of metals and neurodevelopment: a review of recent evidence on susceptibility factors. Curr
Epidemiol Rep. 2020;7:237–62. Article Google Scholar * Weisskopf MG, Webster TF. Trade-offs of personal vs. more proxy exposure measures in environmental epidemiology. Epidemiol Camb Mass.
2017;28:635–43. Article Google Scholar * Arora M, Bradman A, Austin C, Vedar M, Holland N, Eskenazi B, et al. Determining fetal manganese exposure from mantle dentine of deciduous teeth.
Environ Sci Technol. 2012;46:5118–25. Article CAS Google Scholar * Arora M, Hare D, Austin C, Smith DR, Doble P. Spatial distribution of manganese in enamel and coronal dentine of human
primary teeth. Sci Total Environ. 2011;409:1315–9. Article CAS Google Scholar * DeSimone L, Hamilton PA. Quality of water from domestic wells in principal aquifers of the United States
1991–2004. 2009. https://pubs.usgs.gov/circ/circ1332/includes/circ1332.pdf (Accessed 18 Apr 2020). * Iyare PU. The effects of manganese exposure from drinking water on school-age children: a
systematic review. NeuroToxicology. 2019;73:1–7. Article CAS Google Scholar * McMahon PB, Belitz K, Reddy JE, Johnson TD. Elevated manganese concentrations in United States groundwater,
role of land surface–soil–aquifer connections. Environ Sci Technol. 2019;53:29–38. Article CAS Google Scholar * Rodrigues EG, Bellinger DC, Valeri L, Sharif O, Hasan I, Quamruzzaman Q, et
al. Neurodevelopmental outcomes among 2-to 3-year-old children in Bangladesh with elevated blood lead and exposure to arsenic and manganese in drinking water. Environ Health. 2016;15:44.
https://doi.org/10.1186/s12940-016-0127-y. * Pekkanen J, Pearce N. Environmental epidemiology: challenges and opportunities. Environ Health Perspect. 2001;109:1–5. CAS PubMed PubMed
Central Google Scholar * Claus Henn B, Ogneva-Himmelberger Y, Denehy A, Randall M, Cordon N, Basu B, et al. Integrated assessment of shallow-aquifer vulnerability to multiple contaminants
and drinking-water exposure pathways in Holliston. Mass Water Switz. 2017;10:1–22. Google Scholar * Austin C, Richardson C, Smith D, Arora M. Tooth manganese as a biomarker of exposure and
body burden in rats. Environ Res. 2017;155:373–9. Article CAS Google Scholar * Arora M, Hare DJ. Tooth lead levels as an estimate of lead body burden in rats following pre- and neonatal
exposure. RSC Adv. 2015;5:67308–14. Article CAS Google Scholar * Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, et al. Epidemiologic evaluation of measurement data in the
presence of detection limits. Environ Health Perspect. 2004;112:1691–6. Article CAS Google Scholar * Austin C, Smith TM, Bradman A, Hinde K, Joannes-Boyau R, Bishop D, et al. Barium
distributions in teeth reveal early life dietary transitions in primates. Nature. 2013;498:216–9. Article CAS Google Scholar * Bauer JA, Claus Henn B, Austin C, Zoni S, Fedrighi C, Cagna
G, et al. Manganese in teeth and neurobehavior: sex-specific windows of susceptibility. Environ Int. 2017;108:299–308. Article CAS Google Scholar * Claus Henn B, Austin C, Coull BA,
Schnaas L, Gennings C, Horton MK, et al. Uncovering neurodevelopmental windows of susceptibility to manganese exposure using dentine microspatial analyses. Environ Res. 2018;161:588–98.
Article CAS Google Scholar * Horton MK, Hsu L, Claus Henn B, Margolis A, Austin C, Svensson K, et al. Dentine biomarkers of prenatal and early childhood exposure to manganese, zinc and
lead and childhood behavior. Environ Int. 2018;121:148–58. Article CAS Google Scholar * Arbuckle TE, Liang CL, Morisset A-S, Fisher M, Weiler H, Cirtiu CM, et al. Maternal and fetal
exposure to cadmium, lead, manganese and mercury: the MIREC study. Chemosphere. 2016;163:270–82. Article CAS Google Scholar * Kopp RS, Kumbartski M, Harth V, Brüning T, Käfferlein HU.
Partition of metals in the maternal/fetal unit and lead-associated decreases of fetal iron and manganese: an observational biomonitoring approach. Arch Toxicol. 2012;86:1571–81. Article CAS
Google Scholar * Zota AR, Ettinger AS, Bouchard M, Amarasiriwardena CJ, Schwartz J, Hu H, et al. Maternal blood manganese levels and infant birth weight. Epidemiology. 2009;20:367–73.
Article Google Scholar * Claus Henn B, Bellinger DC, Hopkins MR, Coull BA, Ettinger AS, Jim R, et al. Maternal and cord blood manganese concentrations and early childhood neurodevelopment
among residents near a mining-impacted superfund site. Environ Health Perspect. 2017;125:067020. https://doi.org/10.1289/EHP925. * Krachler M, Rossipal E, Micetic-Turk D. Concentrations of
trace elements in sera of newborns, young infants, and adults. Biol Trace Elem Res. 1999;68:121–35. Article CAS Google Scholar * Agency for Toxic Substances and Disease Registry.
Toxicological profile for manganese. Zotero. 2012; https://www.atsdr.cdc.gov/toxprofiles/tp151.pdf. (Accessed 14 Dec 2019). * Agency for Toxic Substances and Disease Registry. Toxicological
profile for barium and barium compounds. 2007; https://www.atsdr.cdc.gov/toxprofiles/tp24.pdf. (Accessed 14 Feb 2021). * Agency for Toxic Substances and Disease Registry. Toxicological
profile for lead. 2020; https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf. (Accessed 12 Feb 2021). * von Stackelberg K, Guzy E, Chu T, Claus Henn B. Exposure to mixtures of metals and
neurodevelopmental outcomes: a review. Risk Anal. 2015;35:971–1016. Article Google Scholar * Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin
Neurobiol. 1998;8:321–9. Article CAS Google Scholar * Rodrigues EG, Kile M, Dobson C, Amarasiriwardena C, Quamruzzaman Q, Rahman M, et al. Maternal-infant biomarkers of prenatal exposure
to arsenic and manganese. J Expo Sci Environ Epidemiol. 2015;25:639–48. Article CAS Google Scholar * Röllin HB, Kootbodien T, Theodorou P, Odland JØ. Prenatal exposure to manganese in
South African coastal communities. Environ Sci Process Impacts. 2014;16:1903–12. Article Google Scholar * Ljung KS, Kippler MJ, Goessler W, Grandér GM, Nermell BM, Vahter ME. Maternal and
early life exposure to manganese in rural Bangladesh. Environ Sci Technol. 2009;43:2595–601. Article CAS Google Scholar * de Assis Araujo MS, Figueiredo ND, Camara VM, Froes Asmus CIR.
Maternal-child exposure to metals during pregnancy in Rio de Janeiro city, Brazil: The Rio Birth Cohort Study of Environmental Exposure and Childhood Development (PIPA project). Environ Res.
2020;183:109155. Article Google Scholar * Gunier RB, Bradman A, Jerrett M, Smith DR, Harley KG, Austin C, et al. Determinants of manganese in prenatal dentin of shed teeth from CHAMACOS
children living in an agricultural community. Environ Sci Technol. 2013;47:11249–57. https://doi.org/10.1021/es4018688. * Johnston J, Franklin M, Roh H, Austin C, Arora M. Lead and arsenic
in shed deciduous teeth of children living near a lead-acid battery smelter. Environ Sci Technol. 2019;53:6000–6. Article CAS Google Scholar * Balachandran RC, Mukhopadhyay S, McBride D,
Veevers J, Harrison FE, Aschner M, et al. Brain manganese and the balance between essential roles and neurotoxicity. J Biol Chem. 2020;295:6312–29. Article CAS Google Scholar * Oulhote Y,
Mergler D, Bouchard MF. Sex- and age-differences in blood manganese levels in the U.S. general population: National Health and Nutrition Examination Survey 2011-2. Environ Health.
2014;13:87. Article Google Scholar * Zheng W, Kim H, Zhao Q. Comparative toxicokinetics of manganese chloride and methylcyclopentadienyl manganese tricarbonyl (MMT) in Sprague-Dawley rats.
Toxicol Sci J Soc Toxicol. 2000;54:295–301. Article CAS Google Scholar * Mirza FG, Abdul-Kadir R, Breymann C, Fraser IS, Raher A. Impact and management of iron deficiency and iron
deficiency anemia in women’s health. Expert Rev Hematol. 2018;11:727–36. https://doi.org/10.1080/17474086.2018.1502081. * Fitsanakis VA, Zhang N, Garcia S, Aschner M. Manganese (Mn) and iron
(Fe): interdependency of transport and regulation. Neurotox Res. 2010;18:124–31. Article Google Scholar * Tholin K, Sandström B, Palm R, Hallmans G. Changes in blood manganese levels
during pregnancy in iron supplemented and non supplemented women. J Trace Elem Med Biol. 1995;9:13–17. Article CAS Google Scholar * Yoon M, Schroeter JD, Nong A, Taylor MD, Dorman DC,
Andersen ME, et al. Physiologically based pharmacokinetic modeling of fetal and neonatal manganese exposure in humans: describing manganese homeostasis during development. Toxicol Sci.
2011;122:297–316. Article CAS Google Scholar * Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Mol Asp Med. 2005;26:353–62. Article CAS Google Scholar * Wolff NA,
Garrick MD, Zhao L, Garrick LM, Ghio AJ, Thévenod F. A role for divalent metal transporter (DMT1) in mitochondrial uptake of iron and manganese. Sci Rep. 2018;8:211.
https://doi.org/10.1038/s41598-017-18584-4. * Gunier RB, Mora AM, Smith D, Arora M, Austin C, Eskenazi B, et al. Biomarkers of manganese exposure in pregnant women and children living in an
agricultural community in California. Environ Sci Technol. 2014;48:14695–702. Article CAS Google Scholar * National Center For Education Statistics – Students With Disabilities. 2021.
https://nces.ed.gov/programs/coe/indicator/cgg (Accessed 28 May 2021). * Massachusetts Department of Education: Holliston (2020-1). 2020.
https://profiles.doe.mass.edu/profiles/student.aspx?orgcode=01360505&orgtypecode=6 (Accessed 28 May 2021). Download references ACKNOWLEDGEMENTS We are grateful to all the participants of
Holliston and their families for taking part in this study. We thank the staff of the ACHIEVE study including Mayah Burgess, Ursula Svoboda, and Chang Chen. FUNDING The research described
in this paper was funded in part by NIEHS grants: T32ES014562 (AF), F31 ES029010 (JAB); Boston University School of Public Health Early Career Catalyst Award (BCH); NICHD grant R00HD087523
(CA). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Environmental Health, Boston University School of Public Health, Boston, MA, USA Alexa Friedman, Julia Anglen Bauer, Wendy
Heiger-Bernays, Roberta F. White & Birgit Claus Henn * Department of Epidemiology, Geisel School of Medicine, Dartmouth College, Lebanon, NH, USA Julia Anglen Bauer * Department of
Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA Christine Austin & Manish Arora * Department of International Development, Community,
and Environment, Clark University, Worcester, MA, USA Timothy J. Downs * Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA Yorghos Tripodis *
Department of Neurology, Boston University School of Medicine, Boston, MA, USA Roberta F. White Authors * Alexa Friedman View author publications You can also search for this author inPubMed
Google Scholar * Julia Anglen Bauer View author publications You can also search for this author inPubMed Google Scholar * Christine Austin View author publications You can also search for
this author inPubMed Google Scholar * Timothy J. Downs View author publications You can also search for this author inPubMed Google Scholar * Yorghos Tripodis View author publications You
can also search for this author inPubMed Google Scholar * Wendy Heiger-Bernays View author publications You can also search for this author inPubMed Google Scholar * Roberta F. White View
author publications You can also search for this author inPubMed Google Scholar * Manish Arora View author publications You can also search for this author inPubMed Google Scholar * Birgit
Claus Henn View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS AF: conceptualization, writing—original draft, preparation, methodology, formal
analysis. JAB: conceptualization, writing—review and editing, formal analysis. CA: data curation, resources, writing—review and editing. TJD: conceptualization, methodology, writing—review
and editing. YT: supervision, writing—review and editing. WH-B: supervision, writing—review and editing. RFW: supervision, writing—review and editing. MA: data curation, resources,
writing—review and editing. BCH: conceptualization, methodology, supervision, funding acquisition, writing—review and editing. CORRESPONDING AUTHOR Correspondence to Alexa Friedman. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ETHICS APPROVAL All reported studies/experiments with human or animal subjects performed by the authors have been
previously published and complied with all applicable ethical standards (including the Helsinki Declaration and its amendments, institutional/national research committee standards, and
international/national/institutional guidelines). ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY MATERIAL RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative
Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Friedman, A., Bauer, J.A., Austin, C. _et al._ Multiple metals in children’s
deciduous teeth: results from a community-initiated pilot study. _J Expo Sci Environ Epidemiol_ 32, 408–417 (2022). https://doi.org/10.1038/s41370-021-00400-x Download citation * Received:
14 June 2021 * Revised: 21 October 2021 * Accepted: 22 October 2021 * Published: 08 November 2021 * Issue Date: May 2022 * DOI: https://doi.org/10.1038/s41370-021-00400-x SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided
by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Metals * Early-life exposure * Biomonitoring * Child exposure/health







