- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT β-Catenin plays a critical role in cartilage formation and development. To further understand the role of β-catenin in osteoarthritis (OA) development in temporomandibular joint
(TMJ), we have generated _β-catenin_ conditional activation mice (_β-cat_(_ex3_)_Agc1CreER_) by breeding _Agc1-CreER_ mice with _β-catenin__flox_(_ex3_)/_+_ mice. Results of histologic
analysis showed the progressive TMJ defects in 3- and 6-month-old _β-cat_(_ex3_)_Agc1CreER_ mice (tamoxifen induction was performed at 2 weeks of age), including decreased chondrocyte
numbers in the superficial layer associated with less Alcian blue staining, increased numbers of hypertrophic chondrocytes in deep layers, and rough articular surface. Compared to the TMJ
phenotype of _β-cat_(_ex3_)_Col2CreER_ mice, _β-cat_(_ex3_)_Agc1CreER_ mice showed much severe morphological defects in the superficial layer of TMJ. This may reflect that _Agc1-CreER_ mice
could efficiently target cells in the superficial layer of TMJ. Results of immunostaining showed significantly increased expression of MMP13, Col-X, Adamts4, and Adamts5 in TMJ of
_β-cat_(_ex3_)_Agc1CreER_ mice. Results of proliferating cell nuclear antigen (PCNA), Ki67, and terminal deoxinucleotidyl transferase-mediated dUTP-fluorescein nick end labeling (TUNEL)
staining further demonstrated that cell proliferation was decreased and cell apoptosis was increased in condylar cartilage of _β-cat_(_ex3_)_Agc1CreER_ mice. Our findings indicate that
abnormal upregulation of β-catenin in TMJ leads to defects assembling to OA-like phenotype, further demonstrating that β-catenin plays a critical role in TMJ pathogenesis. SIMILAR CONTENT
BEING VIEWED BY OTHERS KINDLIN-2 LOSS IN CONDYLAR CHONDROCYTES CAUSES SPONTANEOUS OSTEOARTHRITIC LESIONS IN THE TEMPOROMANDIBULAR JOINT IN MICE Article Open access 04 July 2022 DIVERGENT
CHONDRO/OSTEOGENIC TRANSDUCTION LAWS OF FIBROCARTILAGE STEM CELL DRIVE TEMPOROMANDIBULAR JOINT OSTEOARTHRITIS IN GROWING MICE Article Open access 25 August 2023 LOXL2 PROMOTES AGGRECAN AND
GENDER-SPECIFIC ANABOLIC DIFFERENCES TO TMJ CARTILAGE Article Open access 19 November 2020 INTRODUCTION The temporomandibular joint (TMJ) is one of the most common sites affected by
osteoarthritis (OA). It has been reported that up to 10 million Americans suffer from TMJ disorders (TMDs) each year and 14.56% of mainland Chinese patients with TMD had radiographic signs
of OA.1,2 Among TMDs, OA is the most prevalent degenerative disease.3 TMJ OA is characterized by cartilage degradation, alterations of subchondral bone remodeling, chronic pain, and joint
dysfunction.4,5 Although TMJ OA is a common degenerative joint disease that affects TMJ cartilage during the aging process, the pathological mechanisms of this disease remain largely
unknown.6 Canonical Wnt/β-catenin signaling plays an important role in the development and progression in multiple forms of arthritis, such as OA,7 spondyloarthritis,8,9,10 and diffuse
idiopathic skeletal hyperostosis.11,12,13 It has been shown that conditional activation of β-catenin in knee joint cartilage and intervertebral disc cartilage leads to knee OA and disc
tissue degeneration.7,14 In most recent studies, we also found that activation of β-catenin signaling in facet joint also causes severe OA-like phenotype (unpublished data). Our goal is to
have comprehensive understanding of the role of Wnt/β-catenin signaling in the pathogenesis of arthritis. TMJ OA is one of the important forms of OA and is a common dental disease. The
pathological progression of TMJ OA is considered to be a similar disease as knee OA.15 In previous studies, we generated _β-cat_(_ex3_)_Col2CreER_ mouse model and demonstrated that
dysregulation of β-catenin causes OA-like cartilage degeneration in the TMJ tissue.16 We suggest that β_-_catenin is a critical molecule in OA pathogenesis. Interestingly, there is no
significant change in the superficial zone of TMJ in _β-cat_(_ex3_)_Col2CreER_ mice. And cell proliferation and apoptosis was not changed upon _β-catenin_ activation in this mouse model.16
TMJ condylar cartilage is comprised of dense extracellular collagen fibers and proteoglycans.17 The condylar cartilage is divided into the superficial, middle, and deep layers.18 The
superficial and/or middle zones of condylar cartilage have been identified as regions enriched with highly proliferative cells.19 Mandibular condylar chondrocyte apoptosis and extracellular
matrix degradation play an important role in the development of cartilage degeneration in TMJ OA.20,21 Moreover, activation of chondrocyte hypertrophy with low metabolism followed by
apoptosis in the condylar cartilage is also considered to be part of the disease pathology associated with condylar cartilage degeneration.22 We propose that the _β-cat_(_ex3_)_Col2CreER_
mice might not be able to fully reveal the pathogenesis of TMJ OA. We have recently examined the targeting specificity and recombination efficiency of _Agc1-CreER__T2_ mice in TMJ tissue and
found that _Agc1-CreER__T2_ mice could efficiently target entire condylar cartilage, including superficial, middle, and deep layers. We decided to use this mouse model to re-evaluate the
functions of β-catenin in TMJ tissue using the new _β-cat_(_ex3_)_Agc1ER_ conditional activation mouse model. It has been suggested that mechanisms of the aggrecan- or collagen-induced
arthritis are very different.23 This may be related to the difference of their expression patterns in the condylar cartilage. Another advantage of using _Agc1-CreER__T2_ mice is that these
mice could target cartilage tissue in adult animals.24 In the present study, we have used _Agc1-CreER__T2_ mice to drive β-catenin overexpression and determined the pathogenesis caused by
β-catenin activation in the TMJ tissue. In our study, we explored whether overexpression of β-catenin in aggrecan_-_expressing chondrocytes could lead to cartilage matrix degradation and
affect cell proliferation and apoptosis, which may contribute to the OA phenotype observed in _β-cat_(_ex3_)_Agc1CreER_ mice. RESULTS HIGH CRE-RECOMBINATION EFFICIENCY AND _Β-CATENIN_
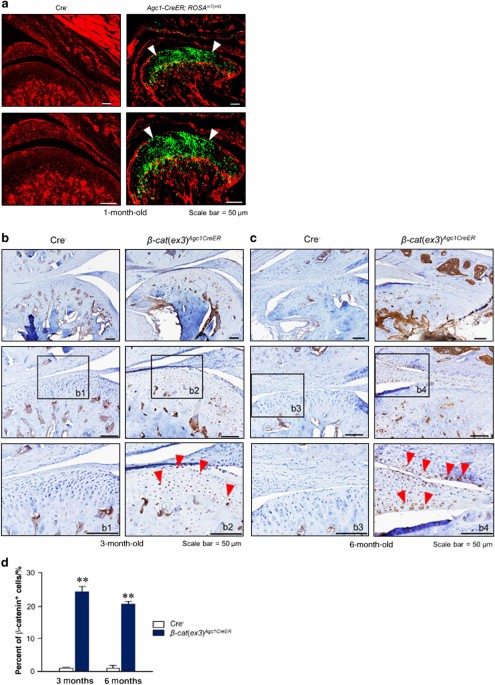
ACTIVATION IN _Β-CAT_(_EX3_)_AGC1CREER_ MICE To evaluate the _Agc1-Cre_ expression and recombination efficiency in the TMJ cartilage, _Agc1-CreER__T2_ mice were bred with _ROSA__mT/mG_
reporter mice to generate _Agc1-CreER__T2__; ROSA__mT/mG_ mice. Tamoxifen was administered when the mice were aged 2 weeks and TMJ samples were harvested at 1 month. The red fluorescent
image of condylar cartilage revealed no recombination in Cre-negative control mice (Fig. 1a). The green-labeled chondrocytes in _Agc1-CreER__T2__; ROSA__mT/mG_ mice showed _Agc1_-expressing
cells in the superficial, middle, and deep layers of condylar chondrocytes (Fig. 1a). We then generated _β-cat_(_ex3_)_Agc1CreER_ mice by crossing _Agc1-CreER__T2_ mice with
_β-catenin_(_ex3_)_flox/flox_ mice. Tamoxifen was administered to 2-week-old mice and condylar cartilage samples were harvested from these mice at 3 and 6 months of age. Immunohistochemical
(IHC) results showed that _β-catenin_ was overexpressed in the majority of condylar chondrocytes at 3- and 6-month-old mice (Fig. 1b, c). There were few β-catenin-positive cells in the
chondrocytes of Cre− mice. However, in _β-cat_(_ex3_)_Agc1CreER_ mice, _β-catenin_ expressed in the superficial, middle, and deep layers of condylar chondrocytes indicating that β-catenin in
the chondrocytes was significantly increased compared to the Cre− mice (Fig. 1b, c). The numbers of _β-catenin_-positive cells in TMJ cartilage were significantly higher in
_β-cat_(_ex3_)_Agc1CreER_ mice compared to Cre− mice (Fig. 1d). These results demonstrated that _Agc1-CreER__T2_ mice could target the chondrocytes of TMJ with high efficiency and drive
β-catenin activation in condylar chondrocytes. CONDITIONAL ACTIVATION OF _Β-CATENIN_ INDUCED CONDYLAR CARTILAGE DEFECTS The role of β-catenin in condylar cartilage was investigated in
_β-cat_(_ex3_)_Agc1CreER_ mice. Tamoxifen was administered to 2-week-old mice and TMJ samples were harvested from these mice at 3 and 6 months of age. The chondrocytes in the control mice
were well organized in the three layers: small and round cells in the top superficial layer; medium-sized cells were present in large numbers in the middle layer; and fewer, bigger,
hypertrophic, mature cells in the deep layer (Fig. 2a, b, left panels). In contrast, 3-month-old _β-cat_(_ex3_)_Agc1CreER_ mice presented early signs of TMJ OA: decreased chondrocyte numbers
in the superficial and middle layer accompanied with less Alcian blue staining in these areas, rough articular surface with numerous rounded chondrocytes often appearing as doublets, and
cells in the middle and deep layers illustrated increased numbers of hypertrophic cells. In addition to decreased cellularity of the middle layers of cartilage, clustering of hypertrophic
chondrocytes appeared more frequently in the deeper layer; cartilage area scattering and subchondral bone sclerosis were also observed in the _β-cat_(_ex3_)_Agc1CreER_ mice compared with
age-matched control group (Fig. 2a, right panel). At 6 months of age, increased severity of defects, such as clustering chondrocytes in the superficial and deeper layer, the increasing
numbers of hypertrophic chondrocytes, and subchondral new bone formation in condylar cartilage were observed in _β-cat_(_ex3_)_Agc1CreER_ mice (Fig. 2b, right panel). We also analyzed the
histology sections using the scoring system recommended by the Osteoarthritis Research Society International (OARSI) as previously described.27,29 We found that _β-cat_(_ex3_)_Agc1CreER_
mice had significantly higher scores for OA damage compared to Cre− mice (Fig. 2c). The histomorphometric analysis also showed significant reductions in articular cartilage area in 3- and
6-month-old _β-cat_(_ex3_)_Agc1CreER_ mice (Fig. 2d). CHANGES IN THE EXPRESSION OF GENES ENCODING FOR MATRIX-DEGRADATION ENZYMES IN _Β-CAT_(_EX3_)_AGC1ER_ MICE We have previously observed
significant upregulation of _Mmp13_ and _Adamts5_ expression in _β-cat_(_ex3_)_Col2CreER_ mice and demonstrated that both matrix metalloproteinase 13 (MMP13) and Adamts5 play important roles
in the TMJ OA development in these mice.16 We propose that _Mmp13_ and _Adamts5_ might be the key downstream target genes of β-catenin during TMJ OA development. To further test this
hypothesis, we performed IHC and immunofluorescence (IF) assays to determine changes in the expression of these collagenase and aggrecanases. Results of IHC revealed increased MMP13
expression in the _β-cat_(_ex3_)_Agc1CreER_ mice, especially in the superficial layer and deeper layer of the condylar cartilage in 3- and 6- month-old mice compared to controls (Fig. 3a,
b). In addition, IF results showed significant increased ColX expression in chondrocytes of entire condylar cartilage in _β-cat_(_ex3_)_Agc1CreER_ mice compared to controls, indicating that
the chondrocytes underwent hypertrophy at this stage (Fig. 4a, b). Furthermore, the expression of cartilage-degrading enzymes, such as Adamts4, and Adamts5 was also increased, especially in
the superficial layer of TMJ chondrocytes in _β-cat_(_ex3_)_Agc1CreER_ mice (Fig. 4c–f). These results suggest that the activation of β-catenin signaling could lead to chondrocyte
hypertrophy and degenerative defects. ALTERATIONS OF CELL PROLIFERATION AND APOPTOSIS IN _Β-CAT_(_EX3_)_AGC1CREER_ MICE To further investigate the pathological process in _β-
cat_(_ex3_)_Agc1CreER_ mice, proliferating cell nuclear antigen (PCNA), Ki67, and terminal deoxinucleotidyl transferase-mediated dUTP-fluorescein nick end labeling (TUNEL) staining was
performed to assess changes in chondrocyte proliferation and apoptosis. In control mice, especially at the 3-month-old, results of PCNA staining showed that abundant proliferating cells were
present in the entire TMJ cartilage. However, the PCNA-positive cells were dramatically reduced in the condylar cartilage of 3- and 6-month-old _β-cat_(_ex3_)_Agc1CreER_ mice (Fig. 5a). To
further analyze changes in cell proliferation, we also performed Ki67 staining and found that numbers of Ki67-positive cells in the middle zones of condylar cartilage were significantly
reduced in 3-month-old _β-cat_(_ex3_)_Agc1CreER_ mice (Fig. 5b, c). These results suggest that overexpression of _β-catenin_ in aggrecan-expressing condylar chondrocytes significantly
affects cell proliferation. Data of TUNEL staining demonstrated the increased apoptotic cells, mostly in the deeper layers of the condylar cartilage of the mutant mice compared to that in
control mice (Fig. 5d, e). In the control group, only few scattered apoptotic cells were detected in the deeper layer of the condylar cartilage in the mice at 6 months of age. Taking
together, these results indicate that conditional activation of _β-catenin_ in the TMJ tissue induced degenerative defects that might be partly due to changes in cell proliferation and
apoptosis. DISCUSSION In this study, we generated _β-cat_(_ex3_)_Agc1CreER_ mouse model and demonstrated that overexpression of _β-catenin_ in aggrecan-expressing chondrocytes leads to
degenerative defects resembling an OA-like phenotype in condylar cartilage. The TMJ OA is a degenerative disease with age-related joint disorder.25 Meanwhile, TMJ disorders mostly affect
young women according to recent researches.26,27 The _Agc1-CreER__T2_ transgenic mouse model is a valuable tool to investigate the postnatal OA development, allowing chondrocyte-specific
gene targeting in an inducible manner.24 To determine the role of β-catenin in TMJ OA development in postnatal mice, we decided to induce β-catenin expression in 2-week-old mice. β-Catenin
was activated specifically in mature chondrocytes. _β-cat_(_ex3_)_Agc1CreER_ mice exhibited TMJ phenotype similar to that of human TMJ OA, including increased chondrocyte hypertrophy
observed in the superficial zone of the condylar cartilage, severe loss of articular cartilage at the margins of cartilage tissue, and subchondral sclerosis. In _β-catenin_ conditional
activation mice, the accelerated catabolic effects (matrix degradation and hypertrophy) may contribute to the eventual loss of the condylar cartilage in this mouse model. In previous
studies, we demonstrated that _β-cat_(_ex3_) _Co12CreER_ mice also showed TMJ OA-like phonotype.16 Compared to middle and deep layers, the superficial area of the condylar cartilage is
relatively normal. This is probably because the _Col2_ gene is not expressed in cells of the superficial layer. _Col2_ is mainly expressed in the middle and deep layers,28 so the condylar
cartilage was not the most efficiently targeted by _Co12-CreER__T2_ mice.3,29,30 In the present study, we specifically determine the role of β-catenin signaling in _Agc1-CreER__T2_ targeting
cells. Interestingly, we observed that activation of β-catenin signaling in aggrecan-expressing cells leads to dramatic damage in the superficial zone of condylar cartilage. This finding
indicates that the superficial zone of the condylar cartilage could be more efficiently targeted by _Agc1-CreER__T2_ mice. Our findings using both transgenic mice clearly demonstrated that
the proper level of β-catenin activity is critical for maintaining the integrity of the condylar cartilage in TMJ. In this study, we also observed significant increases in the expression of
collagenase (MMP13) and aggrecanases (Adamts4 and Adamts5) in _β-cat_(_ex3_)_Agc1CreER_ mice. In addition, there is a significant increase in the expression of ColX, the most specific marker
of hypertrophic chondrocytes, in _β-cat_(_ex3_)_Agc1CreER_ mice. Consistent with this, increased numbers of hypertrophic chondrocytes were observed in the condylar cartilage in
_β-cat_(_ex3_)_Agc1CreER_ mice. MMP13 and Adamts5 are the primary enzymes leading to cartilage degradation.31,32 β-Catenin may serve as an important regulator of MMP13 and Adamts5 in
hypertrophic chondrocytes. In our study, we used two different β-catenin activation mouse models to demonstrate that proper levels of β-catenin are critical in maintaining condylar cartilage
integrity; however, it remains unknown how β-catenin signaling is upregulated during the development of TMJ OA. Previous reports revealed that cell proliferation and apoptosis in condylar
cartilage could also be involved in OA development.20,33 The rat TMJ OA model showed histological changes, including reduced chondrocytes proliferation and increased chondrocytes
apoptosis.34 It has also been reported that the OA is caused by excessive chondrocyte apoptosis.35 The superficial and/or middle zones of normal condylar cartilage have been identified as
regions enriched in cells that are highly proliferative.19 In the present study, in addition to the cartilage degradation we also demonstrated decreased chondrocyte proliferation and
increased chondrocyte apoptosis in _β-cat_(_ex3_)_Agc1CreER_ mice. These changes could also contribute to the development of TMJ OA. The increased numbers of TUNEL-positive chondrocytes may
reflect the enhancement of chondrocyte differentiation in the middle zone of the condylar cartilage in _β-cat_(_ex3_)_Agc1CreER_ mice.36 This is consistent with the notion that cell
apoptosis of mandibular condylar could be responsible for the development and progression of TMJ OA.20,34 In summary, our study revealed that, in addition to changes in the middle and deep
zones, the morphology and function of the superficial zone of cartilage could also be regulated by β-catenin signaling. We suggest that β-catenin may play important roles in chondrocyte
proliferation, differentiation, and apoptosis in the condylar cartilage. Dysregulation of β-catenin signaling in chondrocytes of condylar cartilage may cause significant changes in
chondrocyte function, leading to TMJ OA development. TMJ β-catenin signaling may be served as a potential therapeutic target for the development of drugs to treat TMJ OA. MATERIALS AND
METHODS ANIMALS _Agc1-CreER__T2_ transgenic mice24 and _ROSA__mT/mG_ (membrane-Tomato/membrane-Green) reporter mice37 were obtained from Jackson Laboratories (Bar Harbor, ME, USA).
_β-catenin_(_ex3_)_flox/flox_ mice were originally reported by Harada et al.,38 and we have used these mice in our previous studies.7,14,16 _β-cat_(_ex3_)_Agc1CreER_ mice and the
Cre-negative littermates were generated. Tamoxifen (Sigma, St. Louis, MO, USA) was administered into 2-week-old mice by intraperitoneal (i.p.) injection (1 mg per 10 g body weight for 5
consecutive days), _n_ = 5 in each group. The animal protocol of this study has been approved by the IACUC of the Rush University and all experimental methods and procedures were carried out
in accordance with the approved guidelines. CRE-RECOMBINATION EFFICIENCY _ROSA__mT/mG_ mice contain two _loxP_ sites on either side of the mT cassette. Mice express red fluorescence in all
cell types and tissues before Cre-recombination and green fluorescence signal can be detected after Cre-recombination.37 _Agc1-CreER__T2_ mice were bred with _ROSA__mT/mG_ mice to generate
_Agc1-CreER__T2__; ROSA__mT/mG_ mice. Tamoxifen was administered into 2-week-old mice by i.p. injection (1 mg per 10 g body weight for 5 days). Skulls were dissected after the mice were
sacrificed at age 1 month, fixed in 0.2% glutaraldehyde at 4 °C for 2 days, followed by washing three times with phosphate buffered saline (PBS). Samples were decalcified in 14% EDTA for 3
weeks, cryo-protected in 30% sucrose at 4 °C for 3 days and then embedded and processed for frozen sections. Three-μm-thick sections were imaged with a fluorescence microscope. HISTOLOGY AND
HISTOMORPHOMETRY We dissected the skulls from _β-cat_(_ex3_)_Agc1CreER_ mice and their corresponding Cre-negative control mice. Samples were fixed in 10% neutral buffered formalin (VWR,
Radnor, PA, USA) for 3 days, then decalcified with formic acid (Decal Chemical Corp., Suffern, NY, USA) for 14 days. Samples were processed and embedded in paraffin. Three-μm-thick
mid-sagittal sections at three different levels (50 μm apart) were cut from the medial compartment of the TMJ. The sections were stained with Alcian blue/hematoxylin and eosin for
morphologic analysis. Three slides per mouse, five mice per group, were analyzed in the experiment. The histology data were further analyzed with OARSI scoring system as previously
described.39,40 We also quantified the cartilage area using the OsteoMeasure software (OsteoMetrics, Inc., Atlanta, GA, USA). IHC AND IF The paraffin sections were baked at 65 °C overnight.
Slides were then deparaffinized and rehydrated. Dako endogenous blocking reagent (S2003, Dako, Carpinteria, CA, USA) was then used to quench endogenous peroxidase for 15 min. Non-specific
binding sites were blocked with 1:10 normal horse/goat serum (S-2000, Vector Laboratories, Burlingame, CA, USA) for 30 min at room temperature. Primary antibodies: 1:400 dilution of MMP13
(ab39012, Abcam, Cambridge, UK), 1:1 000 dilution of ColX (ab49945, Abcam, Cambridge, UK); 1:400 dilution of Admts4 (ABT178, Millipore, Billerica, MA), and 1:500 dilution of Adamts5 (ab41037
Abcam, Cambridge, UK) were added, and the slides were incubated at 4 °C overnight. For IHC assays, the secondary biotinylated goat anti-mouse antibody (BA-9200, Vector Laboratories) at the
dilution of 1:200 was added for 30 min on the second day, followed by incubation with 1:250 streptavidin (21130, Pierce, Rockford, IL, USA) for 30 min. Positive staining was detected by
Romulin AEC Chromagen (Biocare Medical RAEC810L, Concord, CA, USA). For IF staining, an appropriate secondary antibody conjugated to a fluorescence probe was added, incubated at room
temperature for 1 h, rinsed in PBS, and mounted in an anti-fading mounting media (Vector Laboratories, Burlingame, CA). Results were obtained using an Olympus BX43 upright microscope
(Olympus Optical, Tokyo, Japan). CELL PROLIFERATION AND APOPTOSIS ASSAYS We dissected TMJ tissues from _β-cat_(_ex3_) _Agc1CreER_ mice and Cre-negative controls. Samples were fixed in 10%
formalin, decalcified, and embedded in paraffin. The condyles were sectioned into serial sections at 3-μm-thick in an anterior–posterior direction. Cell proliferation was carried out using
anti-PCNA and anti-Ki67 antibodies at the dilution of 1:200 (abl8197, ab16667, Abcam, Cambridge, UK) and 1:2 000, respectively, as previously described.16 Apoptosis assay was carried out
using a TUNEL Assay Kit according to the manufacturer’s instructions (G3250, Promega, Madison, WI, USA). STATISTICAL ANALYSIS The values are presented as mean ± standard error. Statistical
difference between groups was evaluated using one-way analysis of variance followed by Tukey–Kramer test and Student’s _t_-test with the SPSS13.0 statistical software. *_P < _0.05 and
**_P_ < 0.01 are considered as significant difference between groups. REFERENCES * Slavkin, H. C. A lifetime of motion: temporomandibular joints. _J. Am. Dent. Assoc._ 127, 1093–1098
(1996). Article PubMed Google Scholar * Zhao, Y. P., Zhang, Z. Y., Wu, Y. T., Zhang, W. L. & Ma, X. C. Investigation of the clinical and radiographic features of osteoarthrosis of the
temporomandibular joints in adolescents and young adults. _Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod._ 111, 27–34 (2011). Article Google Scholar * Chen, M., Li, S., Xie, W.,
Wang, B. & Chen, D. Col2-CreERT2, a mouse model for a chondrocyte-specific and inducible gene deletion. _Eur. Cells Mater._ 28, 236–245 (2014). Article Google Scholar * Kapila, S.,
Wang, W. & Uston, K. Matrix metalloproteinase induction by relaxin causes cartilage matrix degradation in target synovial joints. _Ann. NY Acad. Sci._ 1160, 322–328 (2009). Article
PubMed Google Scholar * Scrivani, S. J., Keith, D. A. & Kaban, L. B. Temporomandibular disorders. _N. Engl. J. Med._ 359, 2693–2705 (2008). Article PubMed Google Scholar * Rando, C.
& Waldron, T. TMJ osteoarthritis: a new approach to diagnosis. _Am. J. Phys. Anthropol._ 148, 45–53 (2012). Article PubMed Google Scholar * Zhu, M. et al. Activation of beta-catenin
signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. _J. Bone Miner. Res._ 24, 12–21 (2009). Article PubMed Google
Scholar * Diarra, D. et al. Dickkopf-1 is a master regulator of joint remodeling. _Nat. Med._ 13, 156–163 (2007). Article PubMed Google Scholar * Appel, H. et al. Altered skeletal
expression of sclerostin and its link to radiographic progression in ankylosing spondylitis. _Arthritis Rheum._ 60, 3257–3262 (2009). Article PubMed Google Scholar * Heiland, G. R. et al.
High level of functional dickkopf-1 predicts protection from syndesmophyte formation in patients with ankylosing spondylitis. _Ann. Rheum. Dis._ 71, 572–574 (2012). Article PubMed Google
Scholar * Kondo, N. et al. Intervertebral disc development is regulated by Wnt/β-catenin signaling. _Spine_ 36, 513–518 (2011). Article Google Scholar * Senolt, L. et al. Low circulating
Dickkopf-1 and its link with severity of spinal involvement in diffuse idiopathic skeletal hyperostosis. _Ann. Rheum. Dis._ 71, 71–74 (2012). Article PubMed Google Scholar * Xie, W.,
Zhou, L., Li, S., Hui, T. & Chen, D. Wnt/β-catenin signaling plays a key role in the development of spondyloarthritis. _Ann. NY Acad. Sci._ 1364, 25–31 (2016). Article PubMed Google
Scholar * Wang, M. et al. Conditional activation of beta-catenin signaling in mice leads to severe defects in intervertebral disc tissue. _Arthritis Rheum._ 64, 2611–2623 (2012). Article
PubMed PubMed Central Google Scholar * Kuroda, S. et al. Biomechanical and biochemical characteristics of the mandibular condylar cartilage. _Osteoarthr. Cartil._ 17, 1408–1415 (2009).
Article PubMed Google Scholar * Wang, M. et al. Activation of beta-catenin signalling leads to temporomandibular joint defects. _Eur. Cells Mater._ 28, 223–235 (2014). Article Google
Scholar * Singh, M. & Detamore, M. S. Tensile properties of the mandibular condylar cartilage. _J. Biomech. Eng._ 130, 011009 (2008). Article PubMed Google Scholar * Mizoguchi, I. et
al. An immunohistochemical study of regional differences in the distribution of type I and type II collagens in rat mandibular condylar cartilage. _Arch. Oral Biol._ 41, 863–869 (1996).
Article PubMed Google Scholar * Hattori, S., Oxford, C. & Reddi, A. H. Identification of superficial zone articular chondrocyte stem/progenitor cells. _Biochem. Biophys. Res. Commun._
358, 99–103 (2007). Article PubMed PubMed Central Google Scholar * Chen, H., Wu, G., Sun, Q., Dong, Y. & Zhao, H. Hyperbaric oxygen protects mandibular condylar chondrocytes from
interleukin-1beta-induced apoptosis via the PI3K/AKT signaling pathway. _Am. J. Transl. Res._ 8, 5108–5117 (2016). PubMed PubMed Central Google Scholar * Loreto, C., Almeida, L. E.,
Trevilatto, P. & Leonardi, R. Apoptosis in displaced temporomandibular joint disc with and without reduction: an immunohistochemical study. _J. Oral Pathol. Med._ 40, 103–110 (2011).
Article PubMed Google Scholar * van der Kraan, P. M. & van den Berg, W. B. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration?
_Osteoarthr. Cartil._ 20, 223–232 (2012). Article PubMed Google Scholar * Hanyecz, A. et al. Proteoglycan aggrecan conducting T cell activation and apoptosis in a murine model of
rheumatoid arthritis. _Biomed. Res. Int._ 2014, 942148 (2014). Article PubMed PubMed Central Google Scholar * Henry, S. P. et al. Generation of aggrecan-CreERT2 knockin mice for
inducible Cre activity in adult cartilage. _Genesis_ 47, 805–814 (2009). PubMed PubMed Central Google Scholar * Guarda-Nardini, L., Piccotti, F., Mogno, G., Favero, L. & Manfredini,
D. Age-related differences in temporomandibular disorder diagnoses. _Cranio_ 30, 103–109 (2012). Article PubMed Google Scholar * Wieckiewicz, M. et al. Prevalence and correlation between
TMD based on RDC/TMD diagnoses, oral parafunctions and psychoemotional stress in Polish university students. _Biomed. Res. Int._ 2014, 472346 (2014). PubMed PubMed Central Google Scholar
* Kogawa, E. M., Calderon, P. D., Lauris, J. R., Pegoraro, L. F. & Conti, P. C. Evaluation of minimum interdental threshold ability in dentate female temporomandibular disorder patients.
_J. Oral Rehabil._ 37, 322–328 (2010). Article PubMed Google Scholar * Hirschmann, P. N. & Shuttleworth, C. A. The collagen composition of the mandibular joint of the foetal calf.
_Arch. Oral Biol._ 21, 771–773 (1976). Article PubMed Google Scholar * Chen, M. et al. Generation of a transgenic mouse model with chondrocyte-specific and tamoxifen-inducible expression
of Cre recombinase. _Genesis_ 45, 44–50 (2007). Article PubMed PubMed Central Google Scholar * Zhu, M., Chen, M., Lichtler, A. C., O’Keefe, R. J. & Chen, D. Tamoxifen-inducible
Cre-recombination in articular chondrocytes of adult Col2a1-CreER(T2) transgenic mice. _Osteoarthr. Cartil._ 16, 129–130 (2008). Article PubMed Google Scholar * Neuhold, L. A. et al.
Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. _J. Clin. Invest._ 107, 35–44 (2001). Article PubMed PubMed
Central Google Scholar * Glasson, S. S. et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. _Nature_ 434, 644–648 (2005). Article PubMed
Google Scholar * Yasuhara, R. et al. Roles of beta-catenin signaling in phenotypic expression and proliferation of articular cartilage superficial zone cells. _Lab. Invest._ 91, 1739–1752
(2011). Article PubMed PubMed Central Google Scholar * Jiao, K. et al. Death and proliferation of chondrocytes in the degraded mandibular condylar cartilage of rats induced by
experimentally created disordered occlusion. _Apoptosis_ 14, 22–30 (2009). Article PubMed Google Scholar * Kim, H. A. & Blanco, F. J. Cell death and apoptosis in osteoarthritic
cartilage. _Curr. Drug Targets_ 8, 333–345 (2007). Article PubMed Google Scholar * Jing, J. et al. Osterix couples chondrogenesis and osteogenesis in post-natal condylar growth. _J. Dent.
Res._ 93, 1014–1021 (2014). Article PubMed PubMed Central Google Scholar * Muzumdar, M. D., Tasic, B., Miyamichi, K., Li, L. & Luo, L. A global double-fluorescent Cre reporter
mouse. _Genesis_ 45, 593–605 (2007). Article PubMed Google Scholar * Harada, N. et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. _EMBO J._ 18,
5931–5942 (1999). Article PubMed PubMed Central Google Scholar * Glasson, S. S., Chambers, M. G., Van Den Berg, W. B. & Little, C. B. The OARSI histopathology initiative
recommendations for histological assessments of osteoarthritis in the mouse. _Osteoarthr. Cartil._ 18, 17–23 (2010). Article Google Scholar * Shen, J. et al. Deletion of the transforming
growth factor beta receptor type II gene in articular chondrocytes leads to a progressive osteoarthritis-like phenotype in mice. _Arthritis Rheum._ 65, 3107–3119 (2013). Article PubMed
PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We would like to express our gratitude to Ms. Lily Yu for her help in processing and staining histological samples. This
work was supported by the National Institutes of Health Grants R01 AR054465 and R01 AR070222 to D.C. This work was also partially supported by the Natural Science Foundation of China (NSFC)
(grant # 81371999) to D.C. T.H. was partially supported by the State Scholarship Fund (No. 201406240061), China. J.L. was partially sponsored by a grant from Shenzhen Science and Technology
Innovation Committee (JCYJ20160331114205502 and JCYJ20150626090344603), and T.W. was partially supported by NSFC grants (grant # 81301531 and 81572104), China. AUTHORS CONTRIBUTIONS D.C.
contributed to the experimental design and data interpretation. T.H., L.Z., S.Z., Y.Z., L.L., T.W., and J.G. carried out all experiments. T.H. contributed to the manuscript preparation. D.C.
and L.Y. contributed to the revision of the manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pediatric Dentistry, Peking University School and Hospital of
Stomatology, Beijing, China Tianqian Hui * State Key Laboratory of Oral Diseases & National Clinical Research Center for Oral Diseases & West China Hospital of Stomatology, Sichuan
University, Chengdu, China Tianqian Hui & Ling Ye * Department of Orthopaedic Surgery, Rush University Medical Center, Chicago, USA Tianqian Hui, Yachuan Zhou, Jun Li, Shanxing Zhang,
Lifan Liao, Jianhong Gu, Lan Zhao & Di Chen * Department of Pharmacy, Shanghai Ninth People’s Hospital, Shanghai JiaoTong University School of Medicine, Shanghai, China Tingyu Wang *
Department of Medical Cell Biology and Genetics, Shenzhen Key Laboratory and the Center for Anti-Ageing and Regenerative Medicine, Shenzhen University Medical School, Shenzhen, China Jun Li
Authors * Tianqian Hui View author publications You can also search for this author inPubMed Google Scholar * Yachuan Zhou View author publications You can also search for this author
inPubMed Google Scholar * Tingyu Wang View author publications You can also search for this author inPubMed Google Scholar * Jun Li View author publications You can also search for this
author inPubMed Google Scholar * Shanxing Zhang View author publications You can also search for this author inPubMed Google Scholar * Lifan Liao View author publications You can also search
for this author inPubMed Google Scholar * Jianhong Gu View author publications You can also search for this author inPubMed Google Scholar * Ling Ye View author publications You can also
search for this author inPubMed Google Scholar * Lan Zhao View author publications You can also search for this author inPubMed Google Scholar * Di Chen View author publications You can also
search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Di Chen. ETHICS DECLARATIONS COMPETING INTEREST The authors declare no competing interests. RIGHTS AND
PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any
medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The
images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not
included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly
from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hui, T., Zhou, Y.,
Wang, T. _et al._ Activation of β-catenin signaling in aggrecan-expressing cells in temporomandibular joint causes osteoarthritis-like defects. _Int J Oral Sci_ 10, 13 (2018).
https://doi.org/10.1038/s41368-018-0016-z Download citation * Accepted: 06 February 2018 * Published: 23 April 2018 * DOI: https://doi.org/10.1038/s41368-018-0016-z SHARE THIS ARTICLE Anyone
you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by
the Springer Nature SharedIt content-sharing initiative







