- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BACKGROUND Obesity often originates in early life, and is linked to excess sugar intake. Nonnutritive sweeteners (NNS) are widely consumed as “healthier” alternatives to sugar, yet
recent evidence suggests NNS may adversely influence weight gain and metabolic health. The impact of NNS during critical periods of early development has rarely been studied. We investigated
the effect of prenatal NNS exposure on postnatal adiposity and adipocyte development. METHODS In the CHILD birth cohort (_N_ = 2298), we assessed maternal NNS beverage intake during
pregnancy and child body composition at 3 years, controlling for maternal BMI and other potential confounders. To investigate causal mechanisms, we fed NNS to pregnant C57BL6J mice at doses
relevant to human consumption (42 mg/kg/day aspartame or 6.3 mg/kg/day sucralose), and assessed offspring until 12 weeks of age for: body weight, adiposity, adipose tissue morphology and
gene expression, glucose and insulin tolerance. We also studied the effect of sucralose on lipid accumulation and gene expression in cultured 3T3-L1 pre-adipocyte cells. RESULTS In the CHILD
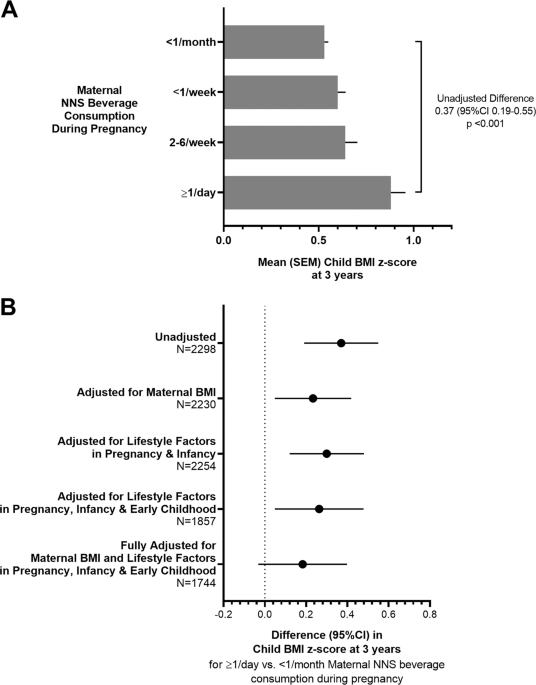
cohort, children born to mothers who regularly consumed NNS beverages had elevated body mass index (mean _z_-score difference +0.23, 95% CI 0.05–0.42 for daily vs. no consumption, adjusted
for maternal BMI). In mice, maternal NNS caused elevated body weight, adiposity, and insulin resistance in offspring, especially in males (e.g., 47% and 15% increase in body fat for
aspartame and sucralose vs. controls, _p_ < 0.001). In cultured adipocytes, sucralose exposure at early stages of differentiation caused increased lipid accumulation and expression of
adipocyte differentiation genes (e.g., C/EBP-α, FABP4, and FASN). These genes were also upregulated in adipose tissue of male mouse offspring born to sucralose-fed dams. CONCLUSION By
triangulating evidence from humans, mice, and cultured adipocytes, this study provides new evidence that maternal NNS consumption during pregnancy may program obesity risk in offspring
through effects on adiposity and adipocyte differentiation. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS
OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on
SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about
institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS ASSOCIATIONS OF MATERNAL NON-NUTRITIVE SWEETENER INTAKE DURING PREGNANCY WITH
OFFSPRING BODY MASS INDEX AND BODY FAT FROM BIRTH TO ADOLESCENCE Article 05 October 2021 MATERNAL HIGH-FAT DIET PROGRAMS WHITE AND BROWN ADIPOSE TISSUE LIPIDOME AND TRANSCRIPTOME IN
OFFSPRING IN A SEX- AND TISSUE-DEPENDENT MANNER IN MICE Article Open access 07 January 2022 WESTERN-STYLE DIET CONSUMPTION IMPAIRS MATERNAL INSULIN SENSITIVITY AND GLUCOSE METABOLISM DURING
PREGNANCY IN A JAPANESE MACAQUE MODEL Article Open access 21 June 2021 REFERENCES * Abarca-Gomez L, Abdeen ZA, Hamid ZA, Abu-Rmeileh NM, Acosta-Cazares B, Acuin C, et al. Worldwide trends in
body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults.
Lancet. 2017;390:2627–42. Google Scholar * Agarwal P, Morriseau TS, Kereliuk SM, Doucette CA, Wicklow BA, Dolinsky VW. Maternal obesity, diabetes during pregnancy and epigenetic mechanisms
that influence the developmental origins of cardiometabolic disease in the offspring. Crit Rev Clin Lab Sci. 2018;55:71–101. CAS PubMed Google Scholar * Borengasser SJ, Zhong Y, Kang P,
Lindsey F, Ronis MJ, Badger TM, et al. Maternal obesity enhances white adipose tissue differentiation and alters genome-scale DNA methylation in male rat offspring. Endocrinology.
2013;154:4113–25. CAS PubMed PubMed Central Google Scholar * Guan H, Arany E, van Beek JP, Chamson-Reig A, Thyssen S, Hill DJ, et al. Adipose tissue gene expression profiling reveals
distinct molecular pathways that define visceral adiposity in offspring of maternal protein-restricted rats. Am J Physiol Endocrinol Metab. 2005;288:E663–73. CAS PubMed Google Scholar *
Liang X, Yang Q, Fu X, Rogers CJ, Wang B, Pan H, et al. Maternal obesity epigenetically alters visceral fat progenitor cell properties in male offspring mice. J Physiol. 2016;594:4453–66.
CAS PubMed PubMed Central Google Scholar * Wen J, Hong Q, Wang X, Zhu L, Wu T, Xu P, et al. The effect of maternal vitamin D deficiency during pregnancy on body fat and adipogenesis in
rat offspring. Sci Rep. 2018;8:365. PubMed PubMed Central Google Scholar * Yang QY, Liang JF, Rogers CJ, Zhao JX, Zhu MJ, Du M. Maternal obesity induces epigenetic modifications to
facilitate Zfp423 expression and enhance adipogenic differentiation in fetal mice. Diabetes. 2013;62:3727–35. CAS PubMed PubMed Central Google Scholar * Hu FB. Resolved: there is
sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes Rev. 2013;14:606–19. CAS PubMed
PubMed Central Google Scholar * Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84:274–88. CAS PubMed PubMed
Central Google Scholar * Seferidi P, Millett C, Laverty AA. Sweetened beverage intake in association to energy and sugar consumption and cardiometabolic markers in children. Pediatr Obes.
2018;13:195–203. CAS PubMed Google Scholar * Vos MB, Kaar JL, Welsh JA, Van Horn LV, Feig DI, Anderson CAM, et al. Added sugars and cardiovascular disease risk in children: a scientific
statement from the American Heart Association. Circulation. 2017;135:e1017–34. CAS PubMed Google Scholar * Fitch C, Keim KS. Position of the Academy of Nutrition and Dietetics: use of
nutritive and nonnutritive sweeteners. J Acad Nutr Diet. 2012;112:739–58. PubMed Google Scholar * Gardner C, Wylie-Rosett J, Gidding SS, Steffen LM, Johnson RK, Reader D, et al.
Nonnutritive sweeteners: current use and health perspectives: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care.
2012;35:1798–808. PubMed PubMed Central Google Scholar * Azad MB, Sharma AK, de Souza RJ, Dolinsky VW, Becker AB, Mandhane PJ, et al. Association between artificially sweetened beverage
consumption during pregnancy and infant body mass index. JAMA. 2016;170:662–70. Google Scholar * Sylvetsky AC, Figueroa J, Rother KI, Goran MI, Welsh JA. Trends in low-calorie sweetener
consumption among pregnant women in the United States. Curr Dev Nutr. 2019;3:nzz004. PubMed PubMed Central Google Scholar * Zhu Y, Olsen SF, Mendola P, Halldorsson TI, Rawal S, Hinkle SN,
et al. Maternal consumption of artificially sweetened beverages during pregnancy, and offspring growth through 7 years of age: a prospective cohort study. Int J Epidemiol. 2017;46:1499–508.
PubMed PubMed Central Google Scholar * Azad MB, Abou-Setta AM, Chauhan BF, Rabbani R, Lys J, Copstein L, et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review
and meta-analysis of randomized controlled trials and prospective cohort studies. Can Med Assoc J. 2017;189:E929–39. Google Scholar * Pearlman M, Obert J, Casey L. The association between
artificial sweeteners and obesity. Curr Gastroenterol Rep. 2017;19:64. PubMed Google Scholar * Toews I, Lohner S, Kullenberg de Gaudry D, Sommer H, Meerpohl JJ. Association between intake
of non-sugar sweeteners and health outcomes: systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. BMJ. 2019;364:k4718. PubMed
PubMed Central Google Scholar * Archibald AJ, Dolinsky VW, Azad MB. Early-life exposure to non-nutritive sweeteners and the developmental origins of childhood obesity: global evidence from
human and rodent studies. Nutrients. 2018;10:194. PubMed Central Google Scholar * Gillman MW, Rifas-Shiman SL, Fernandez-Barres S, Kleinman K, Taveras EM, Oken E. Beverage intake during
pregnancy and childhood adiposity. Pediatrics. 2017;140. https://pediatrics.aappublications.org/content/140/2/e20170031.long. * Araujo JR, Martel F, Keating E. Exposure to non-nutritive
sweeteners during pregnancy and lactation: impact in programming of metabolic diseases in the progeny later in life. Reprod Toxicol. 2014;49:196–201. CAS PubMed Google Scholar * Collison
KS, Makhoul NJ, Zaidi MZ, Saleh SM, Andres B, Inglis A, et al. Gender dimorphism in aspartame-induced impairment of spatial cognition and insulin sensitivity. PLoS ONE. 2012;7:e31570. CAS
PubMed PubMed Central Google Scholar * Olivier-Van Stichelen S, Rother KI, Hanover JA. Maternal exposure to non-nutritive sweeteners impacts progeny’s metabolism and microbiome. Front
Microbiol. 2019;10:1360. PubMed PubMed Central Google Scholar * von Poser Toigo E, Huffell AP, Mota CS, Bertolini D, Pettenuzzo LF, Dalmaz C. Metabolic and feeding behavior alterations
provoked by prenatal exposure to aspartame. Appetite. 2015;87:168–74. Google Scholar * Soffritti M, Belpoggi F, Manservigi M, Tibaldi E, Lauriola M, Falcioni L, et al. Aspartame
administered in feed, beginning prenatally through life span, induces cancers of the liver and lung in male Swiss mice. Am J Ind Med. 2010;53:1197–206. CAS PubMed Google Scholar * Simon
BR, Parlee SD, Learman BS, Mori H, Scheller EL, Cawthorn WP, et al. Artificial sweeteners stimulate adipogenesis and suppress lipolysis independently of sweet taste receptors. J Biol Chem.
2013;288:32475–89. CAS PubMed PubMed Central Google Scholar * Masubuchi Y, Nakagawa Y, Ma J, Sasaki T, Kitamura T, Yamamoto Y, et al. A novel regulatory function of sweet taste-sensing
receptor in adipogenic differentiation of 3T3-L1 cells. PLoS ONE. 2013;8:e54500. CAS PubMed PubMed Central Google Scholar * Pandurangan M, Park J, Kim E. Aspartame downregulates 3T3-L1
differentiation. In Vitro Cell Dev Biol Anim. 2014;50:851–7. CAS PubMed Google Scholar * Subbarao P, Anand SS, Becker AB, Befus AD, Brauer M, Brook JR, et al. The Canadian Healthy Infant
Longitudinal Development (CHILD) Study: examining developmental origins of allergy and asthma. Thorax. 2015;70:998–1000. PubMed Google Scholar * Patterson RE, Kristal AR, Tinker LF, Carter
RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–87. CAS PubMed Google Scholar * Qiu
C, Coughlin KB, Frederick IO, Sorensen TK, Williams MA. Dietary fiber intake in early pregnancy and risk of subsequent preeclampsia. Am J Hypertens. 2008;21:903–9. CAS PubMed Google
Scholar * Maslova E, Strom M, Olsen SF, Halldorsson TI. Consumption of artificially-sweetened soft drinks in pregnancy and risk of child asthma and allergic rhinitis. PLoS ONE.
2013;8:e57261. CAS PubMed PubMed Central Google Scholar * Guenther PM, Casavale KO, Reedy J, Kirkpatrick SI, Hiza HA, Kuczynski KJ, et al. Update of the Healthy Eating Index: HEI-2010. J
Acad Nutr Diet. 2013;113:569–80. PubMed Google Scholar * Pereira TJ, Fonseca MA, Campbell KE, Moyce BL, Cole LK, Hatch GM, et al. Maternal obesity characterized by gestational diabetes
increases the susceptibility of rat offspring to hepatic steatosis via a disrupted liver metabolome. J Physiol. 2015;593:3181–97. CAS PubMed PubMed Central Google Scholar * Dolinsky VW,
Gilham D, Hatch GM, Agellon LB, Lehner R, Vance DE. Regulation of triacylglycerol hydrolase expression by dietary fatty acids and peroxisomal proliferator-activated receptors. Biochim
Biophys Acta. 2003;1635:20–8. CAS PubMed Google Scholar * Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative
RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:Research0034. PubMed PubMed Central Google Scholar * Guariguata L, Linnenkamp U, Beagley J,
Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract. 2014;103:176–85. CAS PubMed Google Scholar * Moseti D, Regassa A, Kim WK.
Molecular regulation of adipogenesis and potential anti-adipogenic bioactive molecules. Int J Mol Sci. 2016;17:E124. PubMed Google Scholar * Roberts A, Renwick AG, Sims J, Snodin DJ.
Sucralose metabolism and pharmacokinetics in man. Food Chem Toxicol. 2000;38 Suppl 2:S31–41. CAS PubMed Google Scholar * Nettleton JE, Reimer RA, Shearer J. Reshaping the gut microbiota:
Impact of low calorie sweeteners and the link to insulin resistance? Physiol Behav. 2016;164:488–93. CAS PubMed Google Scholar * Ruiz-Ojeda FJ, Plaza-Diaz J, Saez-Lara MJ, Gil A. Effects
of sweeteners on the gut microbiota: a review of experimental studies and clinical trials. Adv Nutr. 2019;10 Suppl 1:S31–48. PubMed PubMed Central Google Scholar * Asnicar F, Manara S,
Zolfo M, Truong DT, Scholz M, Armanini F, et al. Studying vertical microbiome transmission from mothers to infants by strain-level metagenomic profiling. mSystems. 2017;2:e00164–16. CAS
PubMed PubMed Central Google Scholar * Le Doare K, Holder B, Bassett A, Pannaraj PS. Mother’s milk: a purposeful contribution to the development of the infant microbiota and immunity.
Front Immunol. 2018;9:361. PubMed PubMed Central Google Scholar * Liou AP, Paziuk M, Luevano JM Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to
gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5:178ra41. PubMed PubMed Central Google Scholar * Meijnikman AS, Gerdes VE, Nieuwdorp M, Herrema H. Evaluating
causality of gut microbiota in obesity and diabetes in humans. Endocr Rev. 2018;39:133–53. PubMed Google Scholar * Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An
obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. PubMed Google Scholar Download references ACKNOWLEDGEMENTS We are grateful to all
the families who took part in the CHILD study, and the whole CHILD team, which includes interviewers, nurses, computer and laboratory technicians, clerical workers, research scientists,
volunteers, managers, and receptionists. We also acknowledge the excellent technical work of Mario Fonseca and Bo Xiang (University of Manitoba), critical review by Shirin Moossavi
(University of Manitoba), and editorial assistance from John Schellenberg (University of Manitoba). A preprint of this work was posted on bioRxiv (https://doi.org/10.1101/713974). FUNDING
The Canadian Institutes of Health Research (CIHR) and the Allergy, Genes and Environment Network of Centers of Excellence (AllerGen NCE) provided core support for the CHILD Study. This
research was supported, in part, by the Canada Research Chairs program. MBA holds the Tier 2 Canada Research Chair in the Developmental Origins of Chronic Disease, and is a Canadian
Institute for Advanced Research Fellow in the Humans and the Microbiome Program. VWD holds the Allen Rouse-Manitoba Medical Services Foundation Basic Scientist Award. MMT is the recipient of
a Research Manitoba/CHRIM studentship. This research was supported by a Children’s Hospital Research Institute of Manitoba Grant, a CIHR Operating Grant #151540, and a CIHR Environments,
Genes and Chronic Disease Team Grant #144626. These entities had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and
preparation, review, or approval of the paper. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Developmental Origins of Chronic Diseases in Children Network (DEVOTION) and the Diabetes
Research Envisioned and Accomplished in Manitoba (DREAM) Theme of the Children’s Hospital Research Institute of Manitoba, Winnipeg, MB, Canada Meghan B. Azad, Alyssa Archibald, Mateusz M.
Tomczyk, Alanna Head, Kyle G. Cheung, Allan B. Becker & Vernon W. Dolinsky * Department of Pediatrics and Child Health, University of Manitoba, Winnipeg, MB, Canada Meghan B. Azad &
Allan B. Becker * Max Rady College of Medicine, University of Manitoba, Winnipeg, MB, Canada Alyssa Archibald * Department of Pharmacology and Therapeutics, University of Manitoba, Winnipeg,
MB, Canada Mateusz M. Tomczyk, Alanna Head, Kyle G. Cheung & Vernon W. Dolinsky * Department of Clinical Epidemiology & Biostatistics, McMaster University, Hamilton, ON, Canada
Russell J. de Souza * Department of Nutritional Sciences, University of Toronto, Toronto, ON, Canada Russell J. de Souza * Li Ka Shing Knowledge Institute, St. Michael’s Hospital, Toronto,
ON, Canada Russell J. de Souza * Department of Pediatrics, University of Alberta, Edmonton, AB, Canada Piushkumar J. Mandhane * Department of Pediatrics, BC Children’s Hospital, University
of British Columbia, Vancouver, BC, Canada Stuart E. Turvey * Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, ON, Canada Theo J. Moraes & Padmaja
Subbarao * Department of Medicine, McMaster University, Hamilton, ON, Canada Malcolm R. Sears Authors * Meghan B. Azad View author publications You can also search for this author inPubMed
Google Scholar * Alyssa Archibald View author publications You can also search for this author inPubMed Google Scholar * Mateusz M. Tomczyk View author publications You can also search for
this author inPubMed Google Scholar * Alanna Head View author publications You can also search for this author inPubMed Google Scholar * Kyle G. Cheung View author publications You can also
search for this author inPubMed Google Scholar * Russell J. de Souza View author publications You can also search for this author inPubMed Google Scholar * Allan B. Becker View author
publications You can also search for this author inPubMed Google Scholar * Piushkumar J. Mandhane View author publications You can also search for this author inPubMed Google Scholar *
Stuart E. Turvey View author publications You can also search for this author inPubMed Google Scholar * Theo J. Moraes View author publications You can also search for this author inPubMed
Google Scholar * Malcolm R. Sears View author publications You can also search for this author inPubMed Google Scholar * Padmaja Subbarao View author publications You can also search for
this author inPubMed Google Scholar * Vernon W. Dolinsky View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS MBA and VWD conceived of the study
design, obtained funding for this research, and drafted the paper. MRS, PS, TJM, SET, PJM, and ABB obtained funding for and oversaw recruitment of the CHILD cohort and data collection. AA
performed the statistical analysis of clinical data from the CHILD cohort under the supervision of MBA. RJS contributed nutritional expertise. MMT, AH, and KGC performed mouse and cell
culture experiments under the supervision of VWD. All authors critically reviewed and approved the paper. MBA had full access to the human data and VWD had full access to the mouse and
adipocyte data, and take final responsibility for the decision to submit for publication. CORRESPONDING AUTHORS Correspondence to Meghan B. Azad or Vernon W. Dolinsky. ETHICS DECLARATIONS
CONFLICT OF INTEREST The authors declare that they have no conflict of interest. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims
in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION REVISED SUPPLEMENT (CLEAN) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Azad, M.B., Archibald, A., Tomczyk, M.M. _et al._ Nonnutritive sweetener consumption during pregnancy, adiposity, and adipocyte differentiation in offspring: evidence from humans, mice, and
cells. _Int J Obes_ 44, 2137–2148 (2020). https://doi.org/10.1038/s41366-020-0575-x Download citation * Received: 14 November 2019 * Revised: 16 March 2020 * Accepted: 27 March 2020 *
Published: 04 May 2020 * Issue Date: October 2020 * DOI: https://doi.org/10.1038/s41366-020-0575-x SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative

