- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT An increase in polymorphonuclear leukocytes (PMNs) and proinflammatory chemokines, such as IL-8 and macrophage inflammatory protein-1α (MIP), are found in the airways during early
stages of bronchopulmonary dysplasia. We determined whether IL-10 produces a dose-related inhibition of proinflammatory chemokine release from stimulated neutrophils of the newborn and
whether the mechanism involves the pivotal transcription factor, nuclear factor-κB. PMNs isolated from the cord blood of healthy newborns were stimulated submaximally with either
lipopolysaccharide (_n_ = 5) or tumor necrosis factor (_n_ = 4), with and without IL-10 (0.01–1000 ng/mL). IL-8 and MIP release were measured in cell culture supernatants at 18 h. The
presence or absence of nuclear factor-κB activity and inhibitor-κBα degradation was measured at 30 min and 3 h after PMN stimulation began. During lipopolysaccharide stimulation, IL-10
significantly reduced IL-8 levels from 50 ± 16 ng/mL to 7 ± 3 ng/mL, and MIP levels from 14 ± 5 to 0.7 ± 0.1 ng/mL (mean ± SEM, _p_ < 0.01). IL-10 produced an insignificant reduction in
IL-8 and MIP levels after stimulation of PMNs with tumor necrosis factor. IL-10 did not inhibit nuclear factor-κB activation and inhibitor-κBα degradation in PMNs stimulated with tumor
necrosis factor or lipopolysaccharide for 30 min. After PMN stimulation for 3 h, inhibitor-κBα cytoplasmic levels were restored; however, they were unaffected by IL-10. We conclude that
IL-10 is a potent inhibitor of lipopolysaccharide-stimulated release of IL-8 and MIP from neutrophils of the newborn via a mechanism not involving nuclear factor-κB activity. Further work is
needed to determine whether exogenous IL-10 may be useful for suppressing inflammation in bronchopulmonary dysplasia. SIMILAR CONTENT BEING VIEWED BY OTHERS DIFFERENTIAL TYPE I INTERFERON
RESPONSE AND PRIMARY AIRWAY NEUTROPHIL EXTRACELLULAR TRAP RELEASE IN CHILDREN WITH ACUTE RESPIRATORY DISTRESS SYNDROME Article Open access 04 November 2020 THE NUCLEAR CYTOKINE IL-37A
CONTROLS LETHAL CYTOKINE STORMS PRIMARILY VIA IL-1R8-INDEPENDENT TRANSCRIPTIONAL UPREGULATION OF PPARΓ Article 27 October 2023 GRANULOCYTE-MACROPHAGE COLONY-STIMULATING FACTOR SUPPRESSES
INDUCTION OF TYPE I INTERFERON IN INFANTS WITH SEVERE PNEUMONIA Article 12 April 2022 MAIN During the first few days of acute lung injury in the newborn, the predominant inflammatory cell
recruited into the lung is the PMN, and the level of influx of these cells is predictive of BPD (1, 2). PMNs are now known to be more than effector cells; they have the ability to amplify
recruitment of PMNs and inflammation by the release of chemokines (3, 4). IL-8 and MIP are two potent chemokines, released by PMNs, that have been detected in the airway fluid of newborns
early in the development of BPD (5, 6). The release of these chemokines is thought to be under the control of a pivotal proinflammatory transcription factor, NF-κB (7). In the PMN, the
unique control of activity and cellular location of NF-κB is now being studied with great intensity (8). In our previous work, using PMNs of the newborn, dexamethasone was shown to inhibit
NF-κB activity and the release of these chemokines (9). There is an urgent need to find safe and effective antiinflammatory therapy, as an alternative to past dexamethasone practices, for
the treatment of BPD (10). IL-10 is a potent antiinflammatory cytokine that is produced by macrophages and possibly neutrophils and other lung cells (11). There is evidence that IL-10 can
suppress many proinflammatory functions of the PMN, but IL-10 may not be produced in sufficient quantity in the newborn to suppress inflammation (12). Therapy with exogenous recombinant
IL-10 has been started experimentally in certain inflammatory diseases of the adult but not yet for the newborn (13). Therefore, the specific aims of the present study were to determine
whether IL-10 is a potent inhibitor of chemokine release from neutrophils of the newborn and whether its mechanism of action was caused by inhibition of NF-κB activity. METHODS SUBJECTS Cord
blood (approximately 30 mL) was obtained from the placenta immediately after elective, cesarean section delivery of 11 term infants, without exposure to general anesthesia. Blood was
collected in heparinized, preservative-free tubes for transport to the laboratory, followed by immediate PMN isolation. The study was approved by the Human Subjects Review Committee of the
North Shore-Long Island Jewish Health Care System. PMN ISOLATION AND CULTURE PROTOCOL PMNs were isolated under endotoxin-free conditions using Ficoll-Paque and dextran (Pharmacia,
Piscataway, NJ, U.S.A.) centrifugation and sedimentation, respectively, followed by hypotonic lysis of the residual erythrocytes as described previously (14). The isolated cells contained
more than 98% PMNs, and the cells were 99% viable as determined by trypan-blue exclusion. Purified cells were resuspended in RPMI 1640 supplemented with 10% FCS (Life Technologies, Grand
Island, NY, U.S.A.) to obtain a final concentration of 5 × 106 cells/mL at 37°C. Cells were exposed to serial doses of purified, recombinant IL-10 (R&D Systems, Minneapolis, MN, U.S.A.)
and incubated at 37°C for 1 h before stimulation with LPS (Sigma Chemical Co., St. Louis, MO, U.S.A.) or recombinant TNF (R&D Systems) for 18 h in polystyrene 96-well culture plates.
Based on previous work from our laboratory, we used submaximal, physiologic doses of TNF (1 ng/mL) and LPS (10 ng/mL) to stimulate the release of IL-8 and MIP (15). We used a near-maximal
inhibitory dose of IL-10, from the dose-response studies above, to assess NF-κB activity (at 30 min) and I-κBα protein expression at 30 min and 3 h after stimulation of PMNs with LPS. These
times were chosen on the basis of previous studies showing NF-κB activation at 30 min (8). We also determined I-κBα expression at 3 h, because a recent study using neutrophils from adults
indicated the effect of IL-10 on neutrophils may need a 3-h exposure (16). MEASUREMENTS ELISA FOR IL-8 AND MIP. Release of IL-8 and MIP from PMNs was measured in cell culture supernatants
using commercially available ELISA kits (R&D Systems) as previously described (3). EMSA FOR NF-ΚB ACTIVITY. Nuclear extracts were prepared from 5 × 106 PMNs for EMSA as previously
described (8, 9). The nuclear extracts were incubated (20 min at room temperature) with 10 fmol of 32P-labeled NF-κB oligonucleotide (5′-TTGTTACAAGGGGACTTTCCGCTGGGGACTTTCCAGGGAG-GC-3′) in 20
μL of binding buffer (20 mM Tris-Cl, pH 7.5, 150 mM KCl, 1 mM EDTA, 1 mM DTT, 0.1% NP-40, 6% glycerol) supplemented with 20 μg of acetylated BSA and 2 μg of poly-deoxyinosine-deoxycytosine.
The resulting complexes were resolved on 5% nondenaturing polyacrylamide gels in 0.5× Tris-Borate-EDTA buffer at 180 V for 2.5 h. The gels were dried and exposed to autoradiographic film
(Kodak Biomax MS; Kodak, Rochester, NY, U.S.A.) with intensifier screen at −80°C overnight. WESTERN ANALYSIS FOR I-ΚBΑ LEVELS. This procedure has been previously described in detail from our
laboratory (8). Cytoplasmic extracts were prepared from PMNs stimulated with TNF or LPS in the presence or absence of IL-10, with separation of proteins on 12% SDS-PAGE, followed by
transfer to a nitrocellulose membrane. The membranes were washed with Tris-buffered saline-Tween (20 mM Tris-Cl, pH 7.6, 1.37 mM NaCl, 0.05% Tween 20), blocked in Tris-buffered saline-Tween
containing 5% nonfat dry milk (Tris-buffered saline-Tween-milk) for 2 h, and incubated 1 h at 25°C with I-κBα primary antibody (SC-371; Santa Cruz Biochemicals, Santa Cruz, CA, U.S.A.)
diluted (1:200) in Tris-buffered saline-Tween-milk. After washing in Tris-buffered saline-Tween, the membranes were incubated (1 h, 25°C) with anti-rabbit secondary antibody conjugated to
horseradish peroxidase and developed using enhanced chemiluminescence detection (Amersham, Piscataway, NJ, U.S.A.). To confirm equal amounts of loaded proteins, the membranes were stripped
and reprobed with actin antibody as described previously (8). STATISTICAL ANALYSES Results are expressed as mean ± SEM. Statistical analyses were performed using ANOVA and the Mann-Whitney
_U_ test, with an overall significance level of 0.05 before Bonferroni correction for multiple comparisons (serial doses of IL-10). RESULTS Figures 1 and 2 demonstrate the effect of serial
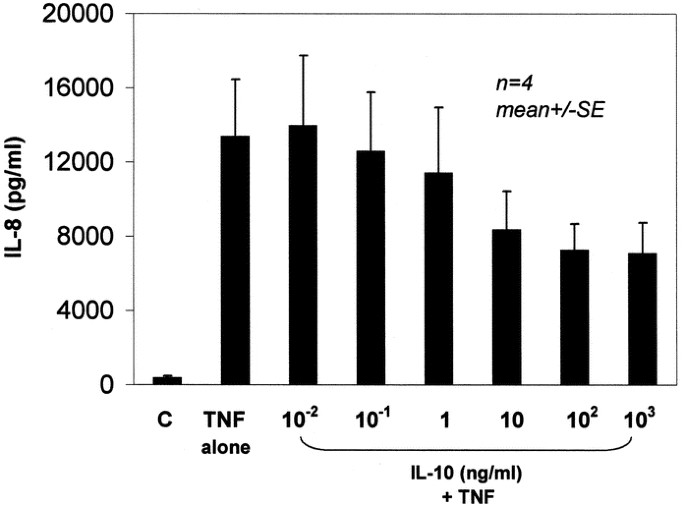
doses of IL-10 on the release of IL-8 and MIP, respectively, from PMNs stimulated with TNF. There was a trend toward inhibition of chemokine release by IL-10, but the results were not
statistically significant for 1, 10, and 100 ng/mL (_p_ > 0.05, _n_ = 4). In contrast, Figures 3 and 4 demonstrate the effect of serial doses of IL-10 on the release of IL-8 and MIP,
respectively, from PMNs stimulated with LPS. There was a statistically significant and marked inhibition of both IL-8 and MIP release with exposure to IL-10 starting at 1 ng/mL (_p_ <
0.01). Therefore IL-10 at a dose of 10 ng/mL was selected as a near-maximal dose for studying the effect of IL-10 on NF-κB activity and I-κBα levels in subsequent experiments. Activation of
NF-κB was directly measured by EMSA and indirectly measured by Western blot analysis of I-κBα levels at simultaneous times before and after stimulation of PMNs with LPS or TNF, and with and
without exposure to IL-10 (10 ng/mL). Figure 5 demonstrates Iκ-Bα levels (_top_) and NF-κB DNA binding (_bottom_) in PMNs stimulated with TNF or LPS for 30 min. The p50/65 and p50/50
subunits of NF-κB are shown; the former heterodimer is the NF-κB component responsible for the transcriptional up-regulation of proinflammatory mediators. Lane 1 demonstrates that before
stimulation with LPS or TNF, there is abundant I-κBα and no NF-κB binding to its oligonucleotide in resting PMNs, indicating NF-κB is not activated. Submaximal doses of TNF or LPS, as shown
in lanes 2 and 4, resulted in a marked reduction in I-κB levels, indicating degradation, and appearance of the NF-κB p50/65 heterodimer indicating activation. Lanes 3 and 5 show that IL-10
had no effect on TNF- or LPS-induced stimulation of NF-κB activity or I-κBα levels. In addition, IL-10 alone did not affect NF-κB activity in resting cells (lane 6). Figure 5 is a
representation of three experiments. To determine whether IL-10 inhibition of chemokines was caused by a delayed inhibition of NF-κB, we examined I-κBα levels with two stimulating doses of
LPS (10 ng and 100 ng) at 30 min (Fig. 6) and 3 h (Fig. 7). The results show that even at lower doses of LPS-induced PMN stimulation (10 and 100 ng/mL), IL-10 did not have any effect on
I-κBα degradation after 30 min (Fig. 6). After 3 h of stimulation with LPS, I-κBα cytoplasmic levels were restored as a result of the NF-κB-induced neosynthesis of I-κBα (Fig. 7). However,
IL-10 again had no significant effect on the resynthesis of new I-κBα. Figures 6 and 7 are representations of one of three experiments for each figure. DISCUSSION Antiinflammatory cytokine
therapy may become a growing field of research in neonatal-perinatal medicine, because of our present lack of safe and effective therapy for diseases such as CLD (10). Neutrophil functions,
long thought to be final effector processes in the acute inflammatory response, now are known to include self-amplifying proinflammatory functions, _e.g._ the release of chemokines (3). Our
present study for the first time demonstrates the dose-related potency of IL-10 in the inhibition of neutrophil chemokine release when neutrophils of the newborn are stimulated by endotoxin.
This inhibition did not occur to the same degree when they were stimulated by TNF; however, had a larger number of studies been performed, a partial inhibition by IL-10 may have been
detectable. Because potential antiinflammatory therapy could be targeted against transcriptional activation of chemokines, we studied NF-κB, a pivotal proinflammatory transcription factor
(7) whose activity is associated with neutrophil chemokine release in the newborn (9). Surprisingly, IL-10 did not inhibit NF-κB activity in the neutrophil of the newborn under our
experimental conditions. Neutrophils are the first inflammatory cells to be recruited into the airways of newborns developing CLD (1, 2). The presence of chemokines such as IL-8 and MIP in
the airway of infants with a high likelihood of developing CLD suggests that resident lung cells and neutrophils recruited into the lung initiate the acute inflammatory response (5, 6).
There are now four classes of chemokines as defined by positioning of cysteine; IL-8 is part of the CXC chemokine class and MIP is part of the CC chemokine class. Accordingly, our
experiments evaluated neutrophil release of two structurally different chemokines that have clinical relevance and are known to be potent stimulants of neutrophil chemotaxis in lung diseases
of the newborn (5, 6). In the present study we found that when neutrophils were stimulated with LPS, a nearly maximal inhibition of chemokine release was observed with IL-10 at a dose of 1
ng/mL. Previous studies have examined IL-10 concentrations in bronchoalveolar lavage fluid from preterm infants ventilated for respiratory distress syndrome. In one study IL-10 was not
detectable in the lungs of preterm infants (12). In a recent study, IL-10 concentrations rose in bronchoalveolar lavage from premature infants up to 5 d after birth; concentrations in the
range of 0.1 to 1 ng/mL were detected (17). Both previous studies indicate that because of low levels of endogenous IL-10, for a short-lived period of time, premature infants with CLD may
lack the antiinflammatory effect provided by IL-10. Extrapolating our _in vitro_ data suggests that supplementing with exogenous IL-10 to blood or alveolar levels in the 1-ng/mL range may
hypothetically provide antiinflammatory protection to infants developing CLD. However, the following questions arise. First, do monocytes/macrophages or other airway cells of the newborn
respond similarly to neutrophils with respect to IL-10 inhibition of chemokine release? And second, how important is TNF (18) as a stimulator of chemokine release in the airways of premature
infants developing CLD? Although IL-10 did not inhibit TNF-induced chemokine release from neutrophils, our previous work demonstrated that dexamethasone did, at concentrations that are
probably much lower than achieved when standard dexamethasone regimens are used clinically for the treatment of CLD (15). It is unknown whether IL-10 and dexamethasone have synergistic
effects on chemokine release from cells that can release proinflammatory mediators. There have been no previous studies in the newborn addressing the mechanism by which IL-10 suppresses
proinflammatory chemokine release. During the last decade, increasing evidence has demonstrated that NF-κB is a pivotal transcription factor associated with a variety of proinflammatory
mediators and apoptosis pathways in a variety of cells (7). In the neutrophil, specifically, NF-κB is bound to an inhibitory protein (I-κBα) that is localized in the nucleus (8). I-κBα
phosphorylation results in ubiquitination and rapid degradation of I-κBα by the proteosome, leading to NF-κB binding to DNA (7). In the present studies, we confirmed NF-κB activation with
I-κBα degradation when neutrophils were exposed to LPS or TNF; IL-10 had no effect on NF-κB activation, measured directly by EMSA or measured indirectly by Western blot analysis of I-κBα
degradation, up to 3 h after neutrophil stimulation. By 3 h after stimulation of neutrophils with LPS, I-κBα had been totally resynthesized. IL-10 had no effect on the resynthesis of I-κBα.
This indicates that IL-10 does not regulate NF-κB signaling in neutrophils of the newborn. The mechanisms of IL-10 inhibition of chemokine production by neutrophils need greater
clarification. Studies involving neutrophils from adults indicate that gene transcription, mRNA destabilization, and _de novo_ induction of a repressor protein may be involved (19–21). The
transcriptional control of chemokine expression is complex and appears to involve multiple protein kinase cascades and several transcription factors such as NF-κB and activating protein-1,
as well as the formation of multiprotein complexes, which interact with DNA (21). Recent studies have shown that in human neutrophils from adults, IL-10 induces phosphorylation and
activation of the transcription factor Stat 3, and up-regulates mRNA synthesis of the suppressor of cytokine signaling-3 (SOCS-3) (16, 22). Interestingly, however, the Stat 3 phosphorylation
and SOCS-3 mRNA synthesis are more dependent on the expression levels of IL-10 receptor (IL-10R1), and more significantly enhanced after neutrophil stimulation with LPS for a few hours
(16). Whether there are developmental differences in IL-10 control of chemokine release, which explains why NF-κB activity was unaffected in our experiments, remains to be determined.
Studies from our laboratory have demonstrated that there are developmental differences in IL-8 production (PMNs from newborns releasing IL-8 to a greater degree than PMNs from adults) and
associated NF-κB activity (3, 9). In addition, recent studies have shown maturational differences in the regulation of another transcription factor, activating protein-1 (23, 24), as well as
in the expression of cell-surface receptors (25–27). Endogenous IL-10 can act as a local, antiinflammatory cytokine, limiting inflammation as shown in animal experiments in which there is a
disrupted IL-10 gene or administration of IL-10 antibody, leading to increased neutrophil recruitment, chemokine release, and worsening of disease (28, 29). Conversely, recombinant human
IL-10, delivered s.c., has been shown to be safe and effective in patients with inflammatory disorders such as mild to moderately active Crohn's disease (13). Because there may be a
relative deficiency of IL-10 in CLD, and because IL-10 is a potent inhibitor of chemokine release in the neutrophil of the newborn stimulated by LPS, we suggest that further work should be
directed to the possibility of using exogenous IL-10 in the treatment of CLD. In addition, a better understanding of the underlying mechanisms accounting for antiinflammatory action of IL-10
may also lead to safer and more effective therapy for CLD compared with corticosteroids. ABBREVIATIONS * MIP: macrophage inflammatory protein-1α * TNF: tumor necrosis factor-α * NF-κB:
nuclear factor-κB * I-κBα: inhibitory factor-κBα * LPS: lipopolysaccharide * PMN: polymorphonuclear leukocyte * CLD: chronic lung disease * BPD: bronchopulmonary dysplasia * EMSA:
electrophoretic mobility shift assay REFERENCES * Merritt TA, Cochrane CG, Holcomb K, Bohl B, Hallman M, Strayer D, Edwards DK, Gluck L 1983 Elastase and alpha-1-proteinase inhibitor
activity in tracheal aspirates during respiratory distress syndrome. _J Clin Invest_ 72: 656–666 Article CAS PubMed PubMed Central Google Scholar * Kwong KYC, Jones CA, Cayabyab R,
Lecart C, Khuu N, Rhandhawa I, Hanley JM, Ramanthan R, deLemos RA, Minoo P 1998 The effects of IL-10 on pro-inflammatory cytokine expression (IL-1β and IL-8) in hyaline membrane disease
(HMD). _Clin Immunol Immunopathol_ 88: 105–113 Article CAS PubMed Google Scholar * Zentay Z, Sharaf M, Qadir M, Drafta D, Davidson D 1999 Mechanism for dexamethasone inhibition of
neutrophil migration upon exposure to lipopolysaccharide _in vitro_: role of neutrophil interleukin-8 release. _Pediatr Res_ 46: 406–410 Article CAS PubMed Google Scholar * Strieter RM,
Kasahara K, Allen RM, Standiford TJ, Rolfe MW, Becker FS, Chensue SW, Kunkel SL 1992 Cytokine-induced neutrophil-derived interleukin-8. _Am J Pathol_ 141: 397–407 CAS PubMed PubMed Central
Google Scholar * Munshi UK, Niu JO, Siddiq MM, Parton LA 1997 Elevation of interleukin-8 and interleukin-6 precedes the influx of neutrophils in tracheal aspirates from preterm infants
who develop bronchopulmonary dysplasia. _Pediatr Pulmonol_ 24: 331–336 Article CAS PubMed Google Scholar * Murch SH, Costeloe K, Klein NJ, MacDonald TT 1996 Early production of
macrophage inflammatory protein-1α occurs in respiratory distress syndrome and is associated with poor outcome. _Pediatr Res_ 40: 490–497 Article CAS PubMed Google Scholar * Barnes PJ,
Karin M 1997 Nuclear factor-κB—a pivotal transcription factor in chronic inflammatory diseases. _N Engl J Med_ 336: 1066–1071 Article CAS PubMed Google Scholar * Vancurova I, Miskolci V,
Davidson D 2001 NF- κB activation in TNF α-stimulated neutrophils is mediated by protein kinase C delta: correlation to nuclear IκBα. _J Biol Chem_ 276: 19746–19752 Article CAS PubMed
Google Scholar * Vancurova I, Bellani P, Davidson D 2001 Activation of nuclear factor-kappa B and its suppression by dexamethasone in polymorphonuclear leukocytes: newborn _versus_ adult.
_Pediatr Res_ 49: 257–262 Article CAS PubMed Google Scholar * Thebaud B, Lacaze-Masmonteil T, Watterberg K 2001 Postnatal glucocorticoids in very preterm infants: “the good, the bad, and
the ugly?”. _Pediatrics_ 107: 413–415 Article CAS PubMed Google Scholar * Cassatella MA 1998 The neutrophil: one of the cellular targets of interleukin-10. _Int J Clin Lab Res_ 289:
148–161 Article Google Scholar * Jones CA, Cayabyab RG, Kwong KY, Stotts C, Wong B, Hamdan H, Minoo P, deLemos RA 1996 Undetectable interleukin (IL)-10 and persistent IL-8 expression early
in hyaline membrane disease: a possible developmental basis for the predisposition to chronic lung inflammation in preterm newborns. _Pediatr Res_ 39: 966–975 Article CAS PubMed PubMed
Central Google Scholar * Fedorak RN, Gangl A, Elson CO, Rutgeerts P, Schreiber S, Wild G, Hanauer SB, Kilian A, Cohard M, LeBeaut A, Feagan B 2000 Recombinant human interleukin 10 in the
treatment of patients with mild to moderately active Crohn's disease. _Gastroenterology_ 119: 1473–1482 Article CAS PubMed Google Scholar * Tan ND, Davidson D 1995 Comparative
differences and combined effects of interleukin-8, leukotriene B4, and platelet-activating factor on neutrophil chemotaxis of the newborn. _Pediatr Res_ 38: 11–16 Article CAS PubMed
Google Scholar * Irakam A, Miskolci V, Vancurova I, Davidson D 2002 Dose-related inhibition of pro-inflammatory cytokine release from neutrophils of the newborn by dexamethasone,
betamethasone, and hydrocortisone. _Biol Neonate_ 82: 89–95 Article CAS PubMed Google Scholar * Crepaldi L, Gasperini S, Lapinet JA, Calzetti F, Pinardi C, Liu Y, Zurawski S, de Waal
Malefyt R, Moore KW, Cassatella MA 2001 Up-regulation of IL-10R1 expression is required to render human neutrophils fully responsive to IL-10. _J Immunol_ 167: 2312–2322 Article CAS PubMed
Google Scholar * Beresford MW, Shaw NJ 2002 Detectable IL-8 and IL-10 in bronchoalveolar lavage fluid from preterm infants ventilated for respiratory distress syndrome. _Pediatr Res_ 52:
973–978 Article CAS PubMed Google Scholar * Jonsson B, Tullus K, Brauner A, Lu Y, Noack G 1997 Early increase of TNFα and IL-6 in tracheobronchial aspirate fluid indicator of subsequent
chronic lung disease in preterm infants. _Arch Dis Child_ 77: F198–F201 Article CAS Google Scholar * Kasama T, Strieter RM, Lukacs NW, Burdick MD, Kunkel SL 1994 Regulation of
neutrophil-derived chemokine expression by IL-10. _J Immunol_ 152: 3559–3569 CAS PubMed Google Scholar * Cassatella MA 1998 The neutrophil: one of the cellular targets of interleukin-10.
_Int J Clin Lab Res_ 28: 148–161 Article CAS PubMed Google Scholar * Kracht M, Saklatvala J 2002 Transcriptional and post-transcriptional control of gene expression in inflammation.
_Cytokine_ 20: 91–106 Article CAS PubMed Google Scholar * Cassatella MA, Gasperini S, Bovolenta C, Calzetti F, Vollebregt M, Scapini P, Marchi M, Suzuki R, Suzuki A, Yoshimura A 1999
Interleukin-10 (IL-10) selectively enhances CIS3/SOCS3 mRNA expression in human neutrophils: evidence for an IL-10-induced pathway that is independent of STAT protein activation. _Blood_ 94:
2880–2889 CAS PubMed Google Scholar * Yang G, Madan A, Dennery PA 2000 Maturational differences in hyperoxic AP-1 activation in rat lung. _Am J Physiol Lung Cell Mol Physiol_ 278:
L393–L398 Article CAS PubMed Google Scholar * Yang G, Shegog ML, Dennery PA 2002 Effect of glutathione on lung activator protein-1 activation and heme oxygenase-1 induction in the
immature rat. _Pediatr Res_ 52: 34–39 Article CAS PubMed Google Scholar * Lorant DE, Li W, Tabatabaei N, Garver MK, Albertine KH 1999 P-selectin expression by endothelial cells is
decreased in neonatal rats and human premature infants. _Blood_ 94: 600–609 CAS PubMed Google Scholar * Gessler P, Neu S, Nebe T, Speer CP 1999 Granulocyte colony-stimulating factor
receptor expression on neutrophils of term and preterm neonates with and without signs of infection. _Eur J Pediatr_ 158: 497–500 Article CAS PubMed Google Scholar * Bessler H, Komlos L,
Punsky I, Ntambi JA, Bergman M, Straussberg R, Sirota L 2001 CD14 receptor expression and lipopolysaccharide-induced cytokine production in preterm and term neonates. _Biol Neonate_ 80:
186–192 Article CAS PubMed Google Scholar * Yan XT, Zhuang M, Oakes J, Lausch RN 2001 Autocrine action of IL-10 suppresses pro-inflammatory mediators and inflammation in the
HSV-1-infected cornea. _J Leukoc Biol_ 69: 149–157 CAS PubMed Google Scholar * Gerard C, Bruyns C, Marchant A, Abramowicz D, Vandenabeele P, Delvaux A, Fiers W, Goldman M, Velu T 1993
Interleukin-10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. _J Exp Med_ 177: 547–550 Article CAS PubMed Google Scholar Download
references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Division of Neonatal-Perinatal Medicine, Schneider Children's Hospital, Long Island Jewish Medical Center, the Long Island
Campus for the Albert Einstein College of Medicine, New Hyde Park, 11040, New York, U.S.A. Johny Tryzmel, Veronika Miskolci, Susana Castro-Alcaraz, Ivana Vancurova & Dennis Davidson
Authors * Johny Tryzmel View author publications You can also search for this author inPubMed Google Scholar * Veronika Miskolci View author publications You can also search for this author
inPubMed Google Scholar * Susana Castro-Alcaraz View author publications You can also search for this author inPubMed Google Scholar * Ivana Vancurova View author publications You can also
search for this author inPubMed Google Scholar * Dennis Davidson View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to
Dennis Davidson. ADDITIONAL INFORMATION Funded in part by Forest Pharmaceuticals, Inc. (Grant Program for Fellows). RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE
THIS ARTICLE Tryzmel, J., Miskolci, V., Castro-Alcaraz, S. _et al._ Interleukin-10 Inhibits Proinflammatory Chemokine Release by Neutrophils of the Newborn without Suppression of Nuclear
Factor-κB. _Pediatr Res_ 54, 382–386 (2003). https://doi.org/10.1203/01.PDR.0000077471.36217.6E Download citation * Received: 30 January 2003 * Accepted: 23 April 2003 * Issue Date: 01
September 2003 * DOI: https://doi.org/10.1203/01.PDR.0000077471.36217.6E SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative


![[withdrawn] near miss with a track worker at llandegai tunnel](https://assets.publishing.service.gov.uk/media/6051d710d3bf7f0455a6e604/s960_Llandegai_tunnel.jpg)



:max_bytes(150000):strip_icc():focal(999x0:1001x2)/kelly-clarkson-1-c90b95f430e74a46bfefa16e858971ea.jpg)
