- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT To investigate the difference in heart rate (HR) recovery after exercise between children and young adults, we administered a constant load of light exercise intensity and
progressive treadmill exercise tests to nine children (aged 9 to 12 y, group A) and eight young adults (six male and two female, aged 17 to 21 y, group B) who had a history of Kawasaki
disease without significant coronary arterial lesions. HR after both exercise protocols was analyzed. The low-frequency (LF) and high-frequency (HF) components of HR variability were
measured, and LF/HF was calculated (log LF, log HF, log L/H). Arterial baroreflex sensitivity was assessed by the phenylephrine method. There were no differences between groups A and B in
resting HR, peak HR, peak oxygen uptake, and decreases in systolic blood pressure during the recovery period. HR 1 and 2 min after peak exercise and 1 min after constant-load exercise was
significantly lower in group A than in group B (_p_ < 0.05), and the changes in HR from peak values after both exercise tests were also greater in group A than in group B (_p_ <
0.05–0.01). Although no difference in arterial baroreflex sensitivity was observed, log HF was significantly higher in group A than in group B (_p_ < 0.01), and log L/H was significantly
lower in group A than in group B (_p_ < 0.05). The value of log HF correlated inversely with the decrease in HR immediately after both exercise protocols (_p_ < 0.05–0.01). Although
log L/H correlated with the decrease in HR after peak exercise (_p_ < 0.05–0.0005), the early decline in HR after constant-load exercise did not correlate with log L/H. Arterial
baroreflex sensitivity did not correlate with the decrease in HR at any recovery time. These data suggest that the early phase of HR recovery after light to severe exercise is influenced by
the cardiac parasympathetic nervous activity at rest and that the greater central cholinergic modulation of HR in children than in young adults may be responsible in part for children's
faster HR recovery after exercise. SIMILAR CONTENT BEING VIEWED BY OTHERS SPEED OF HEART RATE CHANGES DURING POSTURAL PROVOCATIONS IN CHILDREN AND ADOLESCENTS Article Open access 24 May
2024 EFFECTS OF CHRONIC CHOLINERGIC STIMULATION ASSOCIATED WITH AEROBIC PHYSICAL TRAINING ON CARDIAC MORPHOFUNCTIONAL AND AUTONOMIC PARAMETERS IN SPONTANEOUSLY HYPERTENSIVE RATS Article Open
access 25 August 2021 EFFECTS OF AEROBIC, RESISTANCE AND CONCURRENT EXERCISE ON PULSE WAVE REFLECTION AND AUTONOMIC MODULATION IN MEN WITH ELEVATED BLOOD PRESSURE Article Open access 12
January 2021 MAIN HR recovery depends on the cardiorespiratory fitness level of the individual and the intensity of exercise (1–3) and is greatly affected by autonomic nervous activity
during the recovery period (4). According to previous studies, HR recovery is faster in children than in adults (3); however, the precise mechanism of the rapid HR decline in children is
unknown. Recently, measurement of HRV has enabled us to estimate cardiac autonomic nervous activity (5) to some extent. If cardiac autonomic nervous activity is related to HR recovery,
analysis of HR recovery should provide important information in estimating cardiac autonomic function for investigators working with pediatric cardiac patients. Therefore, we investigated
the relationship between HR recovery after exercise and cardiac autonomic nervous activity in children, including arterial baroreflex function, which is important in maintaining blood
pressure by regulating HR. We compared the results with those obtained from young adults. METHODS SUBJECTS Seven boys and two girls aged 9 to 12 y (mean, 10.4 y; group A) and six young adult
men and two women aged 17 to 21 y (mean, 19.1 y; group B) participated in this study. According to secondary sex characteristics (genitalia and pubic hair), the patients in group A were all
Tanner stage 1 (6), _i.e._ they were all prepubertal. The Tanner stage of all subjects in group B was stage 5, _i.e._ postpubertal. All 17 subjects were being followed at our institute
because of a history of Kawasaki disease. Mean follow-up period was 12 ± 5 y (5 to 20 y). However, no stenotic coronary arterial lesions or abnormal hemodynamic data were detected at
follow-up cardiac catheterization and selective coronary angiography. Echocardiography and cardiac catheterization studies were performed during the hospital stay, during which all the
subjects performed the exercise tests of the present study. No significant mitral valve regurgitation or abnormal wall motion of the left ventricle was demonstrated in any of the subjects.
Some subjects showed slight dilation of one coronary artery, and others showed regression of previously dilated coronary arteries. The subjects had no history of chronic pulmonary disease
and had normal cardiopulmonary function (Table 1). No abnormal ECG findings were observed in any subject during an exercise test, and they all lived a normal life without any limitations.
All of the subjects had some experience with treadmill exercise testing in outpatient clinics and were familiar with this kind of exercise test. CONSENT Informed consent was obtained from
all subjects or their parents. The present study was approved by the Ethics Committee of the National Cardiovascular Center. PULMONARY FUNCTION TESTS All 17 patients underwent pulmonary
function tests. We measured the vital capacity (L), the percent forced expired volume in 1 s (%) (Spirosift, SP-600, Fukuda Denshi, Tokyo, Japan), functional residual capacity (mL), residual
volume (mL), and total lung capacity (mL) (Ellopse-1000 System, Fukuda Denshi). The vital capacity values are presented as a percent of normal, and the residual volume to total lung
capacity ratio (%) was calculated. EXERCISE PROTOCOL PEAK EXERCISE. The subjects performed a ramp-like progressive exercise test on a treadmill (Q-5000 system, Quinton, Seattle, WA). After a
4-min rest, the patients completed a 3-min warm-up walk at a speed of 1.5 km/h (grade, 0%) and then exercised with progressive intensity until exhausted. After a 30-s walk-down period, the
patients sat on a chair and cardiorespiratory variables during the next 4 min were obtained. In our original protocol, the exercise intensity was increased by 0.7 metabolic units every 30 s
with completion of the incremental part of the exercise test in approximately 10 min. When determining the slope of V˙O2 as a function of work rate according to the equation MATH where V˙
and G are the velocity (km/h) of the belt and its grade (%), respectively (7), we used a value of 3.5 mL·kg−1·min−1 (= 1.0 metabolic unit) as reported in adults. The value was derived from
the resting V˙O2 for a 70-kg 40-y-old male because of difficulty in determining an actual metabolic unit in children. Using this treadmill protocol, we demonstrated a high correlation
between the values of V˙O2 at AT and the lactate threshold, and we established its clinical usefulness for determining the AT and evaluating cardiorespiratory tolerance in patients with
congenital heart disease (8). Endurance time (min) was defined as the duration of ramp exercise of the protocol. Twelve standard ECG leads were placed to monitor HR during exercise testing.
SBP was measured every 2 min during exercise and 1, 2, and 4 min after peak exercise. Because it is difficult to measure blood pressure with a mercury sphygmomanometer during dynamic
exercise, especially in younger patients, we measured SBP by the palpation method. In a preliminary study, SBP obtained by this method during treadmill exercise testing in 12 patients with
cardiac disease correlated with measurements taken using a mercury sphygmomanometer (_r_ = 0.98, _p_ < 0.0001, our unpublished data, 1996). CONSTANT-LOAD EXERCISE. Each subject performed
a 4-min constant-load exercise test on a separate day. After a 3-min standing period, the patients performed a 4-min constant-load walking test at the AT-level exercise intensity and then
immediately sat on a chair, and cardiorespiratory variables during the next 4 min were obtained. Because of difficulty in determining AT-level exercise intensity on a treadmill protocol, the
stage after which the AT had occurred during our 30-s incremental protocol was adopted as the intensity of constant-load exercise. GAS EXCHANGE MEASUREMENTS. Ventilation and gas exchange
were measured by the breath-by-breath method. Each subject breathed through a mask connected to a hot-wire anemometer (Riko AS500, Minato Medical Science, Osaka, Japan) to measure inspired
and expired volume continuously. A mass spectrometer (MG-300, Perkin Elmer, St. Louis) was used for continuous measurements of oxygen and carbon dioxide partial pressures. Two sizes of full
face masks were used: one for children from 120 to 150 cm tall, which had a dead space of 80 mL, and another for subjects taller than 150 cm, which had a dead space of 100 mL. In the
breath-by-breath protocol, derived respiratory parameters including the respiratory rate, tidal volume, minute ventilation, ventilatory equivalents for oxygen and carbon dioxide, and the
respiratory gas exchange ratio were computed in real time and displayed with HR and V˙O2 on a monitor. A personal computer (PC-9801, NEC, Tokyo) was used for data acquisition and storage.
Breath-by-breath data were averaged to provide one data point for each 30-s period. The delay times and response characteristics of the oxygen and carbon dioxide analyzers were carefully
checked before each exercise test. The metabolic rate above which anaerobic metabolism supplements the production of aerobic energy production and leads to lactic acidosis corresponds to the
AT. This threshold was defined as the V˙O2 at which the ventilatory equivalents for oxygen and end-tidal PO2 increased without increases in the ventilatory equivalents for carbon dioxide
and end-tidal PCO2, alternatively determined by the V-slope method (9, 10). ASSESSMENT OF CARDIAC AUTONOMIC NERVOUS ACTIVITY MEASUREMENT OF HRV. After each subject had rested lying down for
10 min in a quiet room, ECG signals were recorded in the supine position for an additional 5 min at a rate of 1000 samples per second. The data were stored in a personal computer (PC-9821
La10, NEC). The recordings were checked visually to ensure that all QRS complexes on the ECG were correctly labeled. The beat-to-beat fluctuations were transformed into frequency domains by
using fast Fourier transformation. The spectral HRV was expressed as a LF component at 0.04 to 0.15 Hz and a HF component at 0.15 to 0.40 Hz. Because the values were skewed toward larger
values, the logarithmic values of the frequency components, log LF, log HF, and log L/H, were used (11). When premature contractions were detected, removal of ectopy was performed (12).
Central parasympathetic efferent activity to the heart is inhibited during inspiration and the inhibitory magnitude diminishes during expiration (13), so the value of log HF represents the
central parasympathetic efferent nervous activity in normal subjects. On the other hand, log LF can be generated by both the α-adrenergic effector mechanism controlling total peripheral
arterial resistance and the consequently relatively slow fluctuation of HR regulated by the arterial baroreflex for the fluctuation of arterial blood pressure. Thus, log LF is an index of
cardiac sympathetic nervous activity as well as baroreflex-mediated cardiac parasympathetic nervous activity (14), and log L/H is considered to mirror sympathovagal balance or to reflect
sympathetic modulation (5). BRS MEASUREMENT. Arterial blood pressure was monitored using a finger photoplethysmograph (Finapres Ohmeda 2300) that was capable of providing accurate and
reproducible beat-to-beat systolic and diastolic values (15). The respiratory patterns (_i.e._ the phases of inspiration and expiration) were monitored using an impedance device positioned
at the midabdomen. To measure the BRS, phenylephrine was administered in increasing doses (1–2 μg/kg) to determine the dose that would increase the SBP by 20 mm Hg or more. After each bolus
injection, the ECG, arterial blood pressure, and respiration were recorded continuously on a multichannel recorder at a speed of 100 mm/s until the peak blood pressure was reached. The R-R
intervals obtained during expiration were plotted against the SBP of the preceding beats on a beat-to-beat basis. A computerized linear fit was performed to establish the linear portion of
the line of best fit; data points beyond the linear portion of the curve were discarded. Only regression lines that had a correlation coefficient of more than 0.80 or that were statistically
significant (_p_ < 0.05) were accepted for analysis. The final slope was obtained by calculating the mean value of at least two measurements; this value (ms/mm Hg) was considered to
represent the BRS (16). The BRS values of the invasive and noninvasive methods have proven to be highly correlated (17). ANALYSIS OF HR AND SBP RECOVERY. Although the HR recovery pattern
after light or severe exercise is thought to be characterized by one or at least two exponential declines (18), it is clinically convenient and useful to measure the absolute HR or to
calculate the change in HR as a function of recovery time when specific conditions, such as patients with cardiac disease, are under consideration. Therefore, values for HR 1, 2, 3, and 4
min after peak exercise and those at 30 s, 1, 2, and 4 min after constant-load exercise were measured, and the changes in HR after peak HR during each exercise protocol were also calculated
(during peak exercise protocol: ΔHR1, 2, 3, and 4, respectively; and during constant-load exercise protocol: ΔHR30, 60, 120, and 240, respectively). The changes in SBP after peak exercise
were also calculated during the peak exercise protocol (ΔSBP1, 2, and 4, respectively). STATISTICAL ANALYSIS. Differences between the two groups in mean cardiorespiratory values during
exercise were assessed by 2-way repeated-measures ANOVA. Differences between the two groups at each exercise intensity were evaluated by unpaired _t_ test, and changes in cardiorespiratory
variables during exercise were evaluated by paired _t_ test. Data are expressed as mean ± SE, and _p_ values < 0.05 were considered significant. RESULTS CARDIOPULMONARY FUNCTION AT REST.
Although lung volume was significantly lower in group A than in group B, no significant difference in percent predicted values for these variables was observed between the groups. No
significant difference between groups A and B was observed either in the left ventricular end-diastolic volume index (mL/m2) or in the ejection fraction of the ventricle. BRS AND HRV. Values
for BRS and HRV in the study groups are summarized in Table 2. No significant difference in BRS was observed between groups A and B. The value of log HF was significantly higher in group A
than in group B (_p_ < 0.01), whereas the value of log L/H was significantly lower in group A than in group B (_p_ < 0.05). CARDIOVASCULAR RESPONSE DURING PROGRESSIVE EXERCISE.
Although there was no difference in change of HR during progressive exercise, SBP was significantly higher in group B than in group A at rest and at peak exercise (Table 3). Because there
was no difference in V˙O2 at the AT and at peak exercise, physical fitness was equal in both groups. HR AND SBP RECOVERY AFTER PEAK EXERCISE. Although there was no difference in peak HR
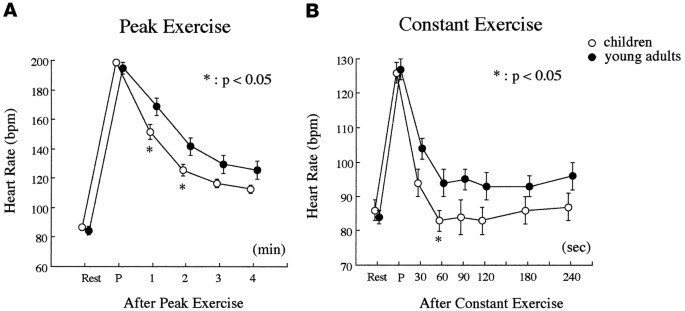
between groups A and B, HR at 1 and 2 min after peak exercise was significantly lower in group A than in group B (Table 4, Fig. 1_a_, _p_ < 0.05), and the changes in HR from peak exercise
to each recovery time were significantly greater in group A than in group B (_p_ < 0.01). Although the values for SBP 1 and 4 min after peak exercise were significantly lower in group A
than in group B, the change in SBP during the recovery period was not different between groups A and B (Table 4). HR AFTER CONSTANT-LOAD EXERCISE. Gas exchange ratio, HR, and V˙O2 were not
different between groups A and B at the end of constant-load exercise (Table 4). The HR values in groups A and B were 125 ± 3 and 126 ± 3, respectively, and these values were not different
from those obtained at the AT exercise intensity during the peak exercise protocol. The V˙O2 values at the end of constant-load exercise in groups A and B were 19.0 ± 0.6 and 20.5 ± 1.3,
respectively, and these two values were not significantly higher than those obtained at AT exercise level during the peak exercise protocol. In addition, because the gas exchange ratios in
groups A and B at the end of the constant-load exercise test were 0.92 ± 0.01 and 0.94 ± 0.02, respectively, the exercise intensity used in the present study did not exceed the intensity of
the AT exercise level. Lower HR values were observed throughout the recovery period in group A compared with those in group B, as demonstrated during peak exercise (Fig. 1_b_). Although only
the HR 60 s after constant-load exercise was significantly lower in group A than in group B, the changes in HR from peak values were significantly greater in group A than in group B
throughout the recovery period (Table 4). The HR values throughout the recovery period were all significantly higher in group B, whereas those values in group A were not significantly
different from HR at rest except at 30 s after the end of constant-load exercise. Conversely, HR 60 s after constant exercise tended to be lower than those at rest and at 180 and 240 s after
the end of constant-load exercise (_p_ < 0.08). CORRELATIONS BETWEEN HRV, BRS, AND DECREASE IN HR AFTEREXERCISE. Correlations between HRV, BRS, and the change in HR after peak and
constant-load exercise testing are summarized in Table 5. BRS did not correlate with the change in HR at any time after either exercise protocol. The log HF value correlated negatively with
all the changes in HR after peak exercise, especially with that 1 min after peak exercise (Fig. 2_a_). On the other hand, the change in HR 30 and 240 s after constant exercise correlated
with log HF (Fig. 2_b_). The value for log L/H also correlated with all the changes in HR after peak exercise, and, the later the period, the stronger the relationship became. However, there
were no significant correlations between log L/H and the changes in HR immediately after constant-load exercise except 240 s after exercise. DISCUSSION HR response during exercise and its
recovery after exercise is regulated by several factors: cardiac autonomic nervous system, increased circulating hormones, elevated body temperature, and mechanical stretch of the heart.
Therefore, HR recovery after exercise depends on the intensity of exercise and the cardiorespiratory fitness of the subject (2, 3). In the present study, because there was no difference in
V˙O2 at the AT and at peak exercise intensity between the two study groups, factors other than physical fitness must affect HR recovery. As mentioned above, because of the influence of
exercise intensity on HR recovery, we used two exercise protocols: AT level and peak exercise tests on a treadmill. Imai _et al._ (19) demonstrated that the time constant for the first 30 s
after exercise was almost independent of exercise intensity and that HR recovery 30 s after exercise, especially at AT exercise intensity, could be a specific index for parasympathetic
nervous-mediated HR recovery. In addition, because the elevation of circulating catecholamines (20, 21) and body temperature is probably small at less than AT exercise intensity, a major
contributing factor to HR recovery is autonomic nervous activity. Actual parasympathetic nervous activity during the early period was not measured in the present study. However, because
ΔHR30 correlated well with log HF, there was probably a tight correlation between parasympathetic nervous activity (tone) at rest and the magnitude of HR recovery early (30–60 s) after light
to moderate exercise. Therefore, it is likely that greater central parasympathetic modulation of HR in children is responsible for the faster HR decrease immediately after AT-level exercise
compared with that in young adults. A persistently elevated level of catecholamines after peak exercise (22) and submaximal exercise has been demonstrated, and it has been thought that this
elevation affects HR recovery after moderate to severe exercise (23). Although the plasma concentration of norepinephrine does not always reflect sympathetic nervous activity, the
relationship between plasma norepinephrine concentration and HR recovery was unclear in the present study because the level of catecholamines was not measured. However, the higher value of
log L/H and the lower magnitude of HR recovery suggest that the relatively high sympathetic nervous activity in young adults creates a significant delay in HR recovery compared with that in
children. It is possible that the change in blood pressure after exercise brings about reflex HR change by the arterial baroreceptors, which are important in attenuating the effects of rapid
change in blood pressure during daily perturbations (24). Therefore, this reflex may have an important role in HR recovery. However, because no significant differences in BRS at rest or
relative change in SBP after peak exercise were observed between children and young adults, it is unclear whether the arterial baroreflex affected HR recovery in the present study.
Walgenbach _et al._ (25, 26) demonstrated that although the arterial baroreflex functioned at the end of exercise to return pressure to normal levels, cardiac output and HR recovery were
almost independent of baroreflex function in conscious dogs. According to these reports, BRS may not be related to HR recovery after exercise. The reason HR recovery is faster in children
than in adults is unclear from the present study. However, fast HR recovery may be necessary for children to prevent excessive cardiac work after exercise. Thus, enhanced central
parasympathetic modulation may play an important role in protecting cardiac overload in children with frequent short intervals of physical activity. BRS is reset to a higher operating point
to permit the arterial blood pressure to increase (27), but it is unknown whether the re-reset of the BRS to a resting level occurs immediately after exercise. However, because HR recovery
after AT exercise level, in which little metabolic changes occur, did not correlate with BRS, it seems that the HR recovery after mild to moderate exercise is not related to the peripheral
arterial baroreflex function. The inhibitory mechanism from the pulmonary stretch receptors to the cardiac parasympathetic nerve, which modifies the HF power of the HRV, must be taken into
consideration when evaluating the parasympathetic activity from the CNS to the heart (13, 28). However, tidal volume to vital capacity ratio at 30 and 60 s after constant-load exercise in
children was not significantly different from that in young adults (30 s: children = 29 ± 2%, young adults = 29 ± 2%; 60 s: children = 21 ± 1%, young adults = 23 ± 2%, NS). Therefore, the
magnitude of inhibition from the pulmonary stretch receptor to cardiac efferent parasympathetic nervous activity in children is approximately the same as in young adults, and thus the
central efferent parasympathetic control of HR is greater in children than that in young adults. STUDY LIMITATIONS. First, our subjects cannot be considered entirely normal. However, no
significant differences in cardiorespiratory response to exercise between patients with a history of Kawasaki disease and normal subjects have been demonstrated, and even patients with
significant coronary arterial lesions showed no significant abnormal findings (29). In our subjects, no stenotic coronary arterial lesions or abnormal hemodynamic parameters were detected by
follow-up cardiac catheterization and selective coronary angiography. Consequently, we believe that our present data are comparable to those from normal healthy subjects. Second, we
estimated the cardiac autonomic nervous activity on the basis of analysis of HRV. Pharmacologic methods, as demonstrated in adult subjects by several investigators, may be preferable, but
this approach is not always feasible in children. High doses of atropine sometimes cause dizziness and/or thirst, which may influence the results to some extent. Parasympathetic nervous
activity estimated by HRV was greater in children than in adults, and this finding is similar to the previously reported results (30). Our study was also limited by the small number of
subjects; however, the data reached statistical significance despite the small sample population. In conclusion, central parasympathetic efferent nervous activity to the heart is greater in
children than in young adults. The greater central cholinergic modulation of HR in children may be responsible in part for children's faster HR recovery, whereas the relatively high
contribution of cardiac sympathetic nervous activity in young adults may account for a significant delay in HR recovery after exercise compared with that in children. ABBREVIATIONS * HR:
heart rate * SBP: systolic blood pressure * HRV: heart rate variability * LF: low-frequency component of HRV * HF: high-frequency component of HRV * L/H: LF to HF ratio of HRV * BRS:
arterial baroreflex sensitivity * V˙O2: oxygen uptake * AT: anaerobic threshold REFERENCES * Hagberg JM, Hickson RC, Ehsani AA, Holloszy JO 1980 Faster adjustment to and recovery from
submaximal exercise in the trained state. _J Appl Physiol_ 48: 218–224 Article CAS Google Scholar * Darr KC, Bassett DR, Morgan BJ, Thomas DP 1988 Effect of age and training status on
heart rate recovery after peak exercise. _Am J Physiol_ 254: H340–H343 CAS PubMed Google Scholar * Baraldi E, Cooper DM, Zanconato S, Armon Y 1991 Heart rate recovery from 1 minute
exercise in children and adults. _Pediatr Res_ 29: 575–579 Article CAS Google Scholar * Savin WM, Davidson DM, Haskell WL 1982 Autonomic contribution to heart rate recovery from exercise
in humans. _J Appl Physiol_ 53: 1572–1575 Article CAS Google Scholar * Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology.
1996 Heart rate variability. _Circulation_ 93: 1043–1065 Article Google Scholar * Tanner JM 1962 Growth at Adolescence, 2nd Ed. Blackwell Scientific Publications, Oxford * Itoh H,
Taniguchi K 1989 Ramp exercise testing for patients with heart failure. _Coronary_ 6: 41–49 CAS Google Scholar * Ohuchi H, Nakajima T, Kawade M, Matsuda H, Kamiya T 1996 Measurement and
validity of the ventilatory threshold in patients with congenital heart disease. _Pediatr Cardiol_ 17: 7–14 Article CAS Google Scholar * Beaver WL, Wasserman K, Whipp BJ 1986 A new method
for detecting anaerobic threshold by gas exchange. _J Appl Physiol_ 60: 2020–2027 Article CAS Google Scholar * Wasserman K, Whipp BJ, Koyal SN, Beaver WL 1973 Anaerobic threshold and
respiratory gas exchange during exercise. _J Appl Physiol_ 35: 236–243 Article CAS Google Scholar * Murakawa Y, Ajiki K, Usui M, Yamashita T, Oikawa N, Inoue H 1993 Parasympathetic
activity is a major modulator of the circadian variability of heart rate in healthy subjects and in patients with coronary artery disease or diabetes mellitus. _Am Heart J_ 126: 108–114
Article CAS Google Scholar * Lipman N, Stein KM, Lerman BB 1994 Comparison of methods for removal of ectopy in measurement of heart rate variability. _Am J Physiol_ 267: H411–H418 Article
Google Scholar * Taha BH, Simon PM, Dempsey JA, Skatrud JB, Iber C 1995 Respiratory sinus arrhythmia in humans: an obligatory role for vagal feedback from the lung. _J Appl Physiol_ 78:
638–645 Article CAS Google Scholar * Madwed JB, Albrecht P, Mark RG, Cohen RJ 1989 Low-frequency oscillations in arterial pressure and heart rate: a simple computer model. _Am J Physiol_
256: H1573–H1579 CAS PubMed Google Scholar * Friedman DB, Jensen FB, Matzen S, Secher NH 1990 Non-invasive blood pressure monitoring during head-up tilt using the Penaz principle. _Acta
Anaesthesiol Scand_ 34: 519–522 Article CAS Google Scholar * Smyth HS, Slight P, Pickering GW 1969 Reflex regulation of arterial pressure during sleep in man. _Circ Res_ 24: 109–121
Article CAS Google Scholar * La Rovere MT, Mortara A, Bigger JT Jr, Marcus FI, Buonamici PG, Colombo E, Grasso S, Hohnloser SH, Nohara R, Schwartz PJ 1993 Reliability of non-invasive
measurement of baroreflex sensitivity after myocardial infarction: ATRAMI, an international multicenter prospective study. _Eur Heart J_ 14: 253abstr Google Scholar * Stegemann J 1981 The
circulatory system and work. In: Skinner JS (ed) _Exercise Physiology: Physiological Basis of Work and Sport_. Year Book, Chicago, IL, pp 122–125 Google Scholar * Imai K, Sato H, Hori M,
Kusuoka H, Ozaki H, Yokoyama H, Takeda H, Inoue M, Kamada T 1994 Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart
failure. _J Am Coll Cardiol_ 24: 1529–1535 Article CAS Google Scholar * Mazzeo RS, Marshall P 1989 Influence of plasma catecholamine on the lactate threshold during graded exercise. _J
Appl Physiol_ 67: 1319–1322 Article CAS Google Scholar * Ohuchi H, Tasato H, Sugiyama H, Arakaki Y, Kamiya T 1998 Responses of plasma norepinephrine and heart rate during exercise in
patients after Fontan operation and patients with residual right ventricular outflow tract obstruction after definitive reconstruction. _Pediatr Cardiol_ 19: 408–413 Article CAS Google
Scholar * Hagberg JM, Hickson RC, McLane JA, Ehsani AA, Winder WW 1979 Disappearance of norepinephrine from the circulation following strenuous exercise. _J Appl Physiol_ 47: 1311–1314
Article CAS Google Scholar * Perini R, Orizio C, Comande A, Castellano M, Beschi M, Veicsteinas A 1989 Plasma norepinephrine and heart rate dynamics during recovery from submaximal
exercise in man. _Eur J Appl Physiol_ 58: 879–883 Article CAS Google Scholar * Guyton AC, Coleman TG, Granger HJ 1972 Circulation: overall regulation. _Annu Rev Physiol_ 34: 13–46 Article
CAS Google Scholar * Walgenbach SC, Shepherd JT 1984 Role of arterial and cardiopulmonary mechanoreceptors in the regulation of arterial pressure during rest and exercise in conscious
dogs. _Mayo Clin Proc_ 59: 467–475 Article CAS Google Scholar * Walgenbach SC, Donald DE 1983 Inhibition by carotid baroreflex of exercise-induced increase in arterial pressure. _Circ
Res_ 52: 253–262 Article CAS Google Scholar * Rowell LB, O'Leary DS 1990 Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. _J Appl Physiol_ 69:
407–418 Article CAS Google Scholar * Arai Y, Saul JP, Albrecht P, Hartley LH, Lilly LS, Cohen RJ, Colucci WS 1989 Modulation of cardiac autonomic activity during and immediately after
exercise. _Am J Physiol_ 256: H132–H141 CAS PubMed Google Scholar * Rhodes J, Hijazi ZM, Marx GR, Fulton DR 1996 Aerobic exercise function of patients with persistent coronary artery
aneurysms secondary to Kawasaki disease. _Pediatr Cardiol_ 17: 226–230 Article CAS Google Scholar * Yeragani VK, Pohl R, Berger R, Balon R, Srinivasan K 1994 Relationship between age and
heart rate variability in supine and standing posture: a study of spectral analysis of heart rate. _Pediatr Cardiol_ 15: 14–20 Article CAS Google Scholar Download references
ACKNOWLEDGEMENTS The authors thank Drs. Peter M. and Setsuko Olley for assistance in preparing the manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pediatrics,
National Cardiovascular Center, Osaka, Japan Hideo Ohuchi, Hiroshi Suzuki, Kenji Yasuda, Yoshio Arakaki, Shigeyuki Echigo & Tetsuro Kamiya Authors * Hideo Ohuchi View author publications
You can also search for this author inPubMed Google Scholar * Hiroshi Suzuki View author publications You can also search for this author inPubMed Google Scholar * Kenji Yasuda View author
publications You can also search for this author inPubMed Google Scholar * Yoshio Arakaki View author publications You can also search for this author inPubMed Google Scholar * Shigeyuki
Echigo View author publications You can also search for this author inPubMed Google Scholar * Tetsuro Kamiya View author publications You can also search for this author inPubMed Google
Scholar RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ohuchi, H., Suzuki, H., Yasuda, K. _et al._ Heart Rate Recovery after Exercise and Cardiac
Autonomic Nervous Activity in Children. _Pediatr Res_ 47, 329–335 (2000). https://doi.org/10.1203/00006450-200003000-00008 Download citation * Received: 05 May 1999 * Accepted: 13 October
1999 * Issue Date: 01 March 2000 * DOI: https://doi.org/10.1203/00006450-200003000-00008 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative







:max_bytes(150000):strip_icc():focal(319x0:321x2)/people_social_image-60e0c8af9eb14624a5b55f2c29dbe25b.png)