- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT We evaluated the involvement of a possible dysfunction of 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) in the fetal growth retardation and poor growth rates of children born
with intrauterine growth retardation (IUGR). Children with IUGR have a nephron deficit and are also at risk of developing cardiovascular diseases, high blood pressure, glucose intolerance,
and dyslipidemia later in life. The major site of 11β-HSD2 production is the kidney and its deficit causes hypertension. We investigated plasma concentrations of cortisol (F) and cortisone
(E) and the F/E ratio in 26 control children and in 40 IUGR children without catch-up growth. We also determined cholesterol, HbA1C, insulin, and glucose levels in plasma. Mean F values were
106 ± 54.2 ng/mL in control children and 114.6 ± 53.2 ng/mL in IUGR children. Mean E values were 19.5 ± 7.1 ng/mL in control children and 17.9 ± 6.85 ng/mL in IUGR children. The mean F/E
ratio for control children was 5.5 ± 1.7. Eight (20%) of the IUGR children (IUGR children of group 1) had high F/E ratios more than 2 SD above the normal mean: 13.15 ± 4.26, (_p_ <
0.0001) as compared to control children, whereas the other 32 children (IUGR children of group 2) had normal F/E ratios: 5.40 ± 1.43 (_p_ = 0.68). Childhood height was significantly lower
for group 1 than group 2 children (-3.63 SD and -2.92 SD, respectively: _p_ < 0.01) and was negatively correlated with the F/E ratio (p < 0.01). Systolic blood pressure was higher for
group 1 (_p_ = 0.005) and for group 2 (_p_ = 0.015) than for control children. The diastolic pressure in IUGR children of group 1 was higher than that in control children (_p_ = 0.013) and
slightly higher than that in group 2 (_p_ = 0.1, ns). Cholesterol concentrations were higher in group 1 than in group 2 (_p_ = 0.029), and controls (_p_ = 0.017) and correlated positively
with F/E (0.02 < _p_ < 0.05). Fasting insulin concentrations were higher in group 1 than in group 2 (ns) and controls (ns). There was no difference in mean fasting glucose
concentrations, or HbA1C between the three groups. Twenty percent of our children with IUGR and poor growth rates had high F/E ratios, suggesting a possible partial 11β-HSD2 deficit. Whether
these children are at high risk of developing cardiovascular diseases as adults remains to be further evaluated. SIMILAR CONTENT BEING VIEWED BY OTHERS THE UTILITY OF IGF1 IN THE EVALUATION
OF PEDIATRIC PATIENTS WITH ENDOGENOUS HYPERCORTISOLEMIA Article 22 November 2023 INSULIN-LIKE GROWTH FACTOR-1 LEVEL IS A POOR DIAGNOSTIC INDICATOR OF GROWTH HORMONE DEFICIENCY Article Open
access 09 August 2021 ADVANCES IN DIFFERENTIAL DIAGNOSIS AND MANAGEMENT OF GROWTH HORMONE DEFICIENCY IN CHILDREN Article 20 August 2021 MAIN 11β-HSD2 deficiency may be congenital AME(1–3),
or acquired, after liquorice administration(4). In patients suffering from this disease, F is not oxidized to E and binds the nonselective type I mineralocorticoid receptor, thereby causing
hypertension and hypokalemic alkalosis. 11β-HSD2 is widely distributed in the fetus, being present in the kidney, lung, brain, adrenal glands, gonads, liver, colon(5), and placenta(6,7).
Later in life, it is mostly produced in the kidney(8). The concentration of circulating corticosteroids is 2 to 10 times higher in the mother than in the fetus(9). Placental 11β-HSD2
converts F into E and thus protects the fetus from F (intoxication): exposure of the fetus to glucocorticoids causes IUGR. Clark _et al._(10) demonstrated that low birth weight neonates
excrete large amounts of glucocorticoids in urine. Pregnant rats given prednisone(11) or dexamethasone(12) give birth to low weight pups. A recent retrospective study by Kitanaka _et
al._(13) showed that most children with congenital 11β-HSD2 deficiency were small for date neonates. In rats, birth weight and placental weight correlate with 11β-HSD2 activity(12). Small
doses of dexamethasone, which do not affect fetal growth, cause hypertension in adult rats(12). Low birth weight is associated with a high prevalence of cardiovascular disease(14,15),
noninsulin-dependent diabetes mellitus(16), and the Metabolic Syndrome or X syndrome (high concentrations of triglycerides, hypertension, and glucose intolerance)(15). Law and Shiell
reviewed published studies including about 66,000 subjects with hypertension(17). They identified a clear relationship between birth weight and blood pressure, with regression coefficients
of 2 to 3 mm Hg/kg in children (95% confidence interval for the change in systolic blood pressure per kg increase in birth weight after adjusting for current size). We report a study to
evaluate 11β-HSD2 activity in children with IUGR. Children with IUGR are at high risk of developing hypertension as adults, and IUGR is associated with subsequent nephron deficit(18–20). The
major site of 11β-HSD2 production is the kidney(8), and therefore some children with IUGR may have a functional partial 11β-HSD2 deficit. We assessed 11β-HSD2 activity by plasma F/E ratio
determination as this is a better index than the THF+allo THF/THE ratio in urine(21,22). We also investigated other cardiovascular risk factors, by assaying cholesterol, fasting insulin,
glucose, HbA1C, and creatinine in this population. METHODS _IUGR CHILDREN._ We selected IUGR children from those consulting for growth retardation at the Trousseau Hospital. We studied 40
children (20 boys and 20 girls) born with intrauterine growth retardation. Their mean age, at the time of the study was 7.4 ± 3.2 y (range: 1.1 to 13.5 y). IUGR was defined as a birth height
at least 2 SD below the mean according to Usher and Mac Lean standards, which are adjusted for gestational age(23). Mean birth height was 45 ± 2 cm or -2.6 ± 0.7 SD and mean birth weight
was 2475 ± 508 g or -1.6 ± 1.5 SD). The children were small for gestational age with a mean gestational age of 38.5 ± 1.8 wk at birth. The children presented poor growth rates, with normal
plasma growth hormone level (GH > 10 µg/L) after provocative testing. Mean height was: -3.1 ± 0.6 SD (range: -4 to -2 SD). Mean systolic blood pressure was 103.5 ± 16.2 mm Hg which is the
70th percentile ± 16 according to height(24) and mean diastolic pressure was 64.4 ± 11.2 mm Hg which is the 71.6th percentile ± 19.7. _CONTROLS._ Normal control children, without growth
retardation, were recruited from subjects consulting Trousseau Hospital as normal volunteers or for endocrine investigations, but were excluded for any endocrine disorder. Twenty-six normal
children were studied as controls (13 boys and 13 girls): mean age 6.2 ± 3.6 y (range 0.9 to 13.6 y). They were normal growing children, not receiving any medication. At birth, their
development was adequate for gestational age: mean gestational age was 39.5 ± 1 wk, mean birth height was: 49.5 ± 2.1 cm or -0.05 ± 0.7 SD, and mean birth weight was 3200 ± 183 g or 0.2 ±
0.7 SD. All investigations conformed to the ethical standards stated by the Helsinki declaration (1975), as revised in Tokyo. _LABORATORY TESTS._ Fasting blood samples were collected from
IUGR children and control children between 8 and 9 AM for cortisol, cortisone, glucose, insulin, cholesterol, and creatinine measurements; HbA1C was only assayed in samples from IUGR
children. Cortisone and cortisol were determined using the same sample, as previously described(22). After extraction by dichloromethane, cortisol and cortisone were separated by
chromatography on Celite minicolumns impregnated with ethyleneglycol. Cortisone and cortisol were assayed in their respective fractions by RIA using in-house rabbit polyclonal anticortisone
antibody 125I-cortisone tracer and proximity scintillation assay reagent, for cortisone, and a commercial cortisol RIA kit for cortisol (Incstar CA1549, obtained from Sorin-Biomedica,
Antony, France). Results were corrected for losses during preparation by adding tritiated cortisone and cortisol to the samples before extraction and chromatography. The performance of the
assay was assessed. Reproducibility was high with only 7.9 to 11.8% variation between serum cortisol assays at concentrations of 7 to 250 ng/mL, and 10.2 to 11.6% variation for cortisone
assays at concentrations of 2.5 to 150 ng/mL (_n_ = 20). The minimum concentration detectable (0 + 3 SD) was 0.9 ng/mL for cortisone, and 2.5 ng/mL for cortisol. There were no measurable
blank values. HbA1C was determined by HPLC, the normal range (mean ± 2 SD) being 3.9 to 5.7% of total Hb. Fasting insulin levels were determined by RIA (INSI-PR - Cis Biointernational).
_STATISTICAL ANALYSIS._ Data were analyzed by a nonparametric test: the Mann-Whitney _U_ test. Values of _p_ < 0.05 were considered to be statistically significant. RESULTS _PLASMA
CORTISOL AND CORTISONE CONCENTRATIONS AND CORTISOL/CORTISONE RATIO._ The mean concentration of F in samples from the 26 control children was: 106 ± 54.2 ng/mL (range 49 to 207 ng/mL) and the
mean E concentration was: 19.5 ± 7.1 ng/mL (range 6.2 to 36.3 ng/mL). The mean F/E ratio was 5.5 ± 1.7 (range 2.66 to 8.97). The F/E ratio in plasma did not differ between age groups or
sexes. For IUGR children, the mean F concentration was 114.6 ± 53.2 ng/mL (range 53.3 to 184.5 ng/mL) (_p_ = 0.28), and mean E concentration was 17.9 ± 6.85 ng/mL (range 5.8 to 32.4 ng/mL)
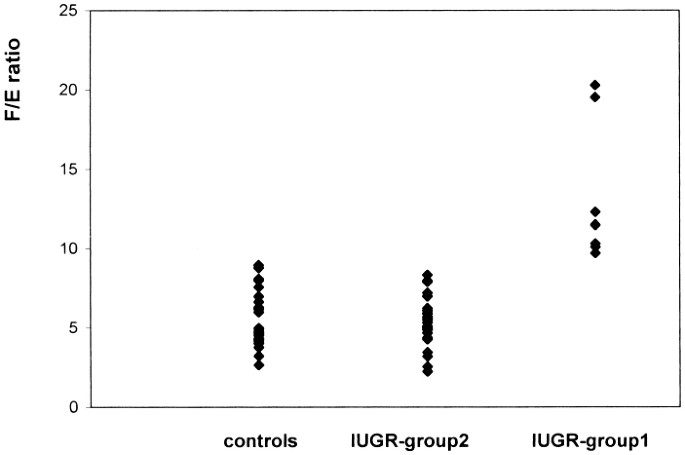
(_p_ = 0.46). The mean F/E ratio in IUGR children was 7.03 ± 3.8 (range 2.23 to 20.3), which was significantly higher than that in control (_p_ = 0.043). We identified two populations among
these 40 children. Eight IUGR children (defined as IUGR children of group 1) had high F/E ratios, more than 2 SD above the control value (13.15 ± 4.26, _p_ < 0.0001). The F/E ratio for
group 1 was significantly higher than that for the other 32 children (IUGR children of group 2) (_p_ < 0.0001). The F/E ratios of IUGR children of group 2 were not significantly different
from those of the control group (5.40 ± 1.43, _p_ = 0.68),(Fig. 1). F values in IUGR children of group 1 were higher than those in IUGR children of group 2 (_p_ = 0.0002), the mean F values
being: 180 ± 56.8 ng/mL and 98.23 ± 38.2 ng/mL, respectively (Fig. 2). E values tended to be lower in group 1 than in group 2, the mean E values being 15.2 ± 7.29 ng/mL and 18.6 ± 6.7
ng/mL, respectively, (_p_ = 0.09) (Fig. 3). _ANTHROPOMETRIC DATA._ Birth heights were lower for group 1 (-3.03 ± 0.9 SD) than for group 2: (-2.56 ± 0.71 SD), but the differences was not
significant (_p_ = 0.35). Birth weights were similar for group 1 (-1.6 ± 1.2 SD) and group 2 (-1.4 ± 1.14 SD)(_p_ = 0.45). Height during childhood was lower for group 1 than group 2 (-3.63
SD _versus_ -2.92 SD; _p_ = 0.005). The children with the slowest growth rate had the highest F/E ratios (Fig. 4). _BLOOD PRESSURE MONITORING._ One patient in group 1 had high blood
pressure. At the age of 6½, his F/E ratio was 10.32. At the age of 12, this patient began to suffer from hypertension, with a mean systolic pressure of 175 mm Hg and diastolic pressure of 75
mm Hg. His height was -3.5 SD below the mean due to IUGR, and his final height was 148 cm. Even excluding this patient from the analysis, mean systolic BP according to height in group 1 was
at the 74th percentile ± 13, in group 2 was at the 69th percentile ± 17 and in the control group at the 48.5th percentile ± 11.8. Thus, both IUGR children groups had significantly higher
systolic blood pressure than control children did, (_p_ = 0.005 for group 1 and was _p_ = 0.015 for group 2). Mean diastolic BP in group 1 was at the 72th percentile ± 13, in group 2 it was
at the 62th percentile ± 9 and in control group at the 48th percentile ± 10. Thus, diastolic BP was higher in group 1 than in control children (_p_ = 0.013), and tended to be higher than in
IUGR children of group 2 although the difference was not significant (_p_ = 0.1). Diastolic BP in group 2 was not significantly different from that of the control group. _CHOLESTEROL
CONCENTRATIONS._ Four of the 8 group 1 children had family histories of high cholesterol levels, or cardiovascular diseases, whereas none of the group 2 children had such family histories.
The mean cholesterol concentration was 5.44 ± 0.35 mmol/L (range: 5.04 to 5.77) in IUGR children of group 1, 4.56 ± 0.94 mmol/L (range: 3.1 to 6.82) in group 2 and 4.56 ± 0.77 mmol/L (range
2.9 to 5.11) in the control group. Thus, cholesterol concentrations in IUGR children of group 1 were higher than those in control group (_p_ = 0.017) and in group 2 (_p_ = 0.029). For IUGR
children (IUGR children of group 1 combined with IUGR children of group 2; _n_ = 40), cholesterol concentration was positively correlated with the F/E ratio (0.02 < _p_ < 0.05) (Fig.
5). _INSULIN, GLUCOSE, AND HBA1C ASSESSMENT._ Fasting insulin levels tended to be higher in IUGR children of group 1 (8.94 ± 4.95 µIU/mL) than in group 2 children (6.45 ± 6.76 µIU/mL), but
the difference was not significant. These values were not significantly higher than control levels (4.8 ± 1.7 µIU/mL). Fasting glucose concentrations were similar for the three groups: 4.55
± 0.625 mmol/L for group 1, 4.76 ± 0.39 mmol/L for group 2 and 4.53 ± 0.36 mmol/L for the control group. HbA1C values were normal in IUGR children 4.86 ± 0.27% (4.5 to 5.3%) for group 1, and
5.12 ± 0.39% (4.6 to 6.35%) for group 2 (_p_ = 0.09). _GLOMERULAR FILTRATION RATE._ The creatinine concentration was normal in all patients: 54 ± 10 µmol/L with a calculated (Schwartz
method) GFR of 96.8 ± 16.1 mL/min ·1.73 m2 in group 1, 54 ± 9.9 µmol/L and a GFR of 98.6 ± 12 mL/min·1.73 m2 in group 2 and 53 ± 10.2 mmol/L with a GFR of 98.9 ± 15 mL/min ·1.73 m2 in the
control group. DISCUSSION Maternal malnutrition, hypoxia, anemia, smoking habits, and alcohol intake affect fetal growth and development(25). Placental 11β-HSD2 activity, which converts
cortisol to cortisone may also affect fetal growth. The F/E ratio is approximately 10:1 in adults and 1:2 in the fetus, so most maternal cortisol arriving at the fetus is inactivated by
conversion into cortisone. Partial functional deficit of 11β-HSD2 activity resulting in high levels of exposure to glucocorticoids _in utero_(26) may cause fetal growth retardation.
Benediktsson _et al._(12) demonstrated a positive correlation between placental 11βHSD2 activity and birth weight and a negative correlation between placental 11β-HSD2 activity and placental
weight in rats. Retrospective data concerning birth weights of patients suffering from congenital 11β-HSD2 deficiency or AME show that a very high proportion of these individuals suffered
from IUGR (17 of 18 AME patients)(13). In AME patients, because 11β-HSD2 is absent, cortisol occupies mineralocorticoid receptors leading to increased sodium resorption, potassium excretion,
and hypertension(1,3). The growth retardation of babies diagnosed as small for gestational age is heterogenous and less than 50% are true IUGR cases. Our selection of IUGR children was
based on birth records confirmed by clinical examination 1 mo after birth. These children were selected when they consulted for growth retardation at the age of 7.4 ± 3.17 y. Twenty percent
(8 of 40) of our population of children with IUGR and no catch-up growth had possible partial deficit of 11β-HSD2 activity, defined as a high (at least 2 SD above the mean) plasma F/E ratio.
The kidney is the main site of cortisone production in man(8). Intrauterine growth retardation in rats is associated with a deficiency in the number of nephrons in fetal rats(18) and in
humans(19,20). The number of glomeruli per optical field is very low in rats with IUGR induced by uterine artery ligation or protein deficiency and in children with IUGR. If birth height is
very low, there may be more than 50% fewer nephrons than normal. Brenner _et al._(27) demonstrated a relationship between nephron loss and high blood pressure in adult life. The Preston UK
study(28) identified the strongest predictor of adult hypertension as being the combination of a low birth weight and a large placenta. This suggests that the smallest fetuses with the
largest placentas may have the lowest 11β-HSD2 activity and the greatest exposure to maternal glucocorticoids. IUGR children are at high risk of developing high blood pressure with
cardiovascular disease, glucose intolerance and resistance to insulin, or the Metabolic Syndrome(14–17). In adults, plasma cortisol concentrations may be as high as 408 nmol/L in men with
low birth weights (2.5 kg or less) and as high as 309 nmol/L in men with birth weights of at least 4.3 kg (_p_ < 0.007)(29). A partial deficit in 11β-HSD2 function may cause high adult
blood pressure in some IUGR patients. In our IUGR children of group 1, systolic blood pressure was significantly higher than that in control children, and diastolic BP was slightly higher
than that in IUGR children of group 2 although the difference was not significant. The F/E ratio in our patients correlated with cholesterol levels and may be a high risk factor for
cardiovascular disease. A correlation between cholesterol and cortisol concentrations has previously been reported, and confirmed by Rubin _et al._(30). It was considered to be a
cardiovascular risk factor because there was a statistically significant correlation between cortisol and cholesterol concentrations in individuals with coronary artery disease but not in
subjects without coronary disease(31). Insulin levels were slightly higher in IUGR children of group 1 than IUGR children of group 2 although the difference was not significant. It has been
suggested(26) that glucocorticoid exposure during critical periods may retard fetal growth and may have irreversible effects leading to hypertension in adult life. The intrauterine
environment and brief changes in hormone concentrations may determine the risk of common disorders throughout the patient's life. The relationship between the plasma F/E ratio and
height in childhood was statistically significant (_p_ < 0.01), suggesting that an F-E ImBalance continues to exert its effects later in childhood. This partial deficit in 11β-HSD2
function may reduce growth due to relative glucocorticoid excess or rather, glucocorticoid imbalance. Indeed, cortisol metabolism and growth hormone levels may be controlled by direct or
indirect regulation pathways(32) not involving changes in cortisol availability. The plasma F/E ratio may be a useful biologic marker for random surveys of IUGR patients. Our results are
preliminary and should be interpreted with caution. A larger number of patients should be studied to produce more accurate conclusions particularly concerning long-term cardiovascular
disease risks. To conclude, we found that 20% of children with IUGR and poor growth rates had high F/E ratios, suggesting a possible partial 11β-HSD2 deficit. These patients also had higher
cholesterol levels and a tendency toward higher insulin levels. They are also shorter and have high diastolic and systolic blood pressure, suggesting that they are at high risk of developing
Metabolic Syndrome including cardiovascular disease and diabetes later in life. ABBREVIATIONS * 11β-HSD2: 11β-hydroxysteroid dehydrogenase type 2 * AME: apparent mineralocorticoid excess *
GFR: glomerular filtration rate * IUGR: intrauterine growth retardation * F: cortisol * E: cortisone REFERENCES * Ulick S, Levine LS, Gunczler P, Zanconato G, Ramirez LC, Rauh W, Rosler A,
Bradlow HL, New W M I 1979 A syndrome of mineralocorticoid excess associated with defects in the peripheral metabolism of cortisol. _J Clin Endocrinol Metab_ 49: 757–764 Article CAS Google
Scholar * Stewart PM, Corrie JET, Shackelton CHL, Edwards CRW 1988 Syndrome of apparent mineralocorticoid excess: a defect in the cortisol-cortisone shuttle. _J Clin Invest_ 82: 340–349
Article CAS Google Scholar * White PC, Mune T, Agarwal AK 1997 11β-Hydroxysteroid dehydrogenase and the syndrome of apparent Mineralocorticoid excess. _Endocrine Rev_ 18: 135–156 CAS
Google Scholar * Stewart PM, Valentino R, Wallace AM, Burt D, Shackelton CHL, Edwards CRW 1987 Mineralocorticoid activity of liquorice: 11 βeta-hydroxysteroid dehydrogenase deficiency comes
of age. _Lancet_ 821: 824 Google Scholar * Stewart PM, Murry BA, Mason JI 1994 Type 2:11 β Hydroxysteroid dehydrogenase in human fetal tissues. _J Clin Endocrinol Metab_ 78: 1529–1532 CAS
PubMed Google Scholar * Krozowski Z, Maguire JA, Stein-Oakley AN, Dowling J, Smith RE, Andrews RK 1995 Immunohistochemical localization of the 11 β-hydroxysteroid dehydrogenase type II
enzyme in human kidney and placenta. _J Clin Endocrinol Metab_ 80: 2203–2209 CAS PubMed Google Scholar * Stewart PM, Rogerson F M, Mason JI 1995 Type 2 11β-hydroxysteroid dehydrogenase
messenger ribonucleic acid and activity in human placenta and fetal membranes: its relationship to birth weight and putative role in fetal adrenal steroidogenesis. _J Clin Endocrinol Metab_
80: 885–890 CAS PubMed Google Scholar * Whitworth JA, Stewart PM, Burt D, Atherden SM, Edwards CRW 1989 The kidney is the major site of cortisone production in man. _Clin Endocrinol_ 31:
355–361 Article CAS Google Scholar * Beitins IZ, Bayard F, Ance SIV, Kowarski A, Migeon CJ 1973 The metabolic clearance rate, blood production, interconversion and transplacental passage
of cortisol and cortisone in pregnancy near term. _Pediatr Res_ 7: 509–519 Article CAS Google Scholar * Clark PM, Hindmarsh PC, Shiell AW, Law CM, Honour JW, Barker DJP 1996 Size at birth
and adrenocortical function in childhood. _Clin Endocrinol_ 45: 721–726 Article CAS Google Scholar * Reinisch JM, Simon NG, Karow WG, Gandelman R 1978 Prenatal exposure to prednisone in
humans and animals retards intrauterine growth. _Science_ 436: 438 Google Scholar * Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CRW 1993 Glucocorticoid exposure in utero: new
model for adult hypertension. _Lancet_ 341: 339–341 Article CAS Google Scholar * Kitanaka S, Tanae A, Hibi I 1996 Apparent mineralocorticoid excess due to 11 β hydroxysteroid
dehydrogenase deficiency: a possible cause of intrauterine growth retardation. _Clin Endocrinol_ 44: 353–359 Article CAS Google Scholar * Barker DJP, Osmond C, Golding J, Kuh D, Wadsworth
MEJ 1989 Growth in utero, blood pressure in childhood and adult life and mortality from cardiovascular disease. _BMJ_ 298: 564–567 Article CAS Google Scholar * Barker DJP, Gluckman PD,
Godfrey KM, Harding JE, Owens JA, Robinson JS 1993 Fetal nutrition and cardiovascular disease in adult life. _Lancet_ 341: 938–941 Article CAS Google Scholar * Hales CN, Barker DJP, Clark
PMS, Cox LJ, Fall C, Osmond C, Winter PD 1991 Fetal and infant growth and impaired glucose tolerance at age 64. _BMJ_ 303: 1019–1022 Article CAS Google Scholar * Law CH, Shiell AW 1996
Is blood pressure inversely related to birthweight? The strength of evidence from a systematic review of the literature. _J Hypertens_ 14: 935–941 Article CAS Google Scholar *
Merlet-Benichou C, Gilbert T, Muffat-Joly M, Lelièvre-Pégorier M, Leroy B 1994 Intrauterine growth retardation leads to a permanent nephron deficit in the rat. _Pediatr Nephrol_ 8: 175–180
Article CAS Google Scholar * Hinchliffe SA, Lynch MRJ, Sargent PH, Howard CV, van Velzen D 1992 The effect of intrauterine growth retardation on the development of renal nephrons. _Br J
Obstet Gynaecol_ 99: 296–301 Article CAS Google Scholar * Merlet-Benichou C, Vilar J, Lelièvre-Pegorier M, Moreau E, Gilbert T 1997 Fetal nephron mass: its control and deficit. _Adv
Nephrol Necker Hosp_ 26: 19–45 CAS PubMed Google Scholar * Palermo M, Shackelton CHL, Mantero F, Stewart PH 1995 Urinary free cortisone and the assessment of 11 β hydroxysteroid
dehydrogenase activity in man. _Clin Endocrinol_ 45: 605–611 Article Google Scholar * Morineau G, Boudi A, Barka A, Gourmelen M, Degeilh F, Hardy N, Al-Halnak A, Soliman H, Gosling JP,
Julien R, Brerault JL, Boudou P, Aubert P, Villette JM, Pruna A, Galons H, Fiet J 1997 Radioimmunoassay of cortisone in serum, urine, and saliva to assess the status of the
cortisol-cortisone shuttle. _Clin Chem_ 43: 1397–1407 CAS PubMed Google Scholar * Usher R, Mac Lean F 1969 Intrauterine growth of live-born Caucasian infants at sea level: Standards
obtained from measurements in 7 dimensions of infants born between 25 and 44 wk of gestation. _J Pediatr_ 74: 901–910 Article CAS Google Scholar * National Heart, Lung and Blood Institute
Bethesda. MD: 1987 Report of the second task force on blood pressure control in children. _Pediatrics_ 79: 1–25 * Wollmann H A 1998 A 1998 Intrauterine growth restriction: definition and
etiology. _Horm Res_ 49( suppl 2): 1–6 Article CAS Google Scholar * Edwards CRW, Benediktsson R, Lindsay RS, Seckl JR 1993 Dysfunction of placental glucocorticoid barrier: link between
fetal environment and adult hypertension?. _Lancet_ 341: 355–357 Article CAS Google Scholar * Brenner BM, Garcia DL, Anderson S 1988 Glomeruli and blood pressure, less of one, more of the
other?. _Am J Hypertens_ 1: 335–345 Article CAS Google Scholar * Barker DJP, Bull AR, Osmond C, Simmonds SJ 1991 Fetal and placental size and risk of hypertension in adult life. _BMJ_
302: 1205–1237 Article Google Scholar * Phillips DIW, Barker DJP, Fall CHD, Seckl JR, Whorwood CS, Wood PJ, Walker BR 1998 Elevated plasma cortisol concentrations: a link between low birth
weight and the insulin resistance syndrome. _J Clin Endocrinol Metab_ 83: 757–760 CAS PubMed Google Scholar * Rubin RT, Clark BR, Poland RE, Arthur RJ 1971 Serum uric acid, cholesterol,
and cortisol intercorrelations in normoactive subjects. _Am Heart J_ 81: 843–845 Article CAS Google Scholar * Schwertner HA, Troxler RG, Uhl GS, Jackson WG 1984 Relationship between
cortisol and cholesterol in men with coronary artery disease and type A behavior. _Atherosclerosis_ 4: 59–64 CAS Google Scholar * Gelding SV, Taylor NF, Wood PJ, Noonan K, Weaver JU, Wood
DF, Monson JP 1998 The effect of growth hormone replacement therapy on cortisol-cortisone interconversion in hypopituitary adults: evidence for growth hormone modulation of extrarenal 11
β-hydroxysteroid dehydrogenase activity. _Clin Endocrinol_ 48: 153–162 Article CAS Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Laboratoire
d'Explorations Fonctionnellles Endocriniennes, Hopital Trousseau AP-HP, 26 avenue du Dr Arnold Netter, 75012, Paris Muriel Houang, Gilles Morineau, Yves le Bouc, Jean Fiet &
Micheline Gourmelen * Laboratoire de Biologie Hormonale, Hopital St. Louis AP-HP, 1 Avenue Claude Vellefaux, Paris, 75010, France Muriel Houang, Gilles Morineau, Yves le Bouc, Jean Fiet
& Micheline Gourmelen Authors * Muriel Houang View author publications You can also search for this author inPubMed Google Scholar * Gilles Morineau View author publications You can also
search for this author inPubMed Google Scholar * Yves le Bouc View author publications You can also search for this author inPubMed Google Scholar * Jean Fiet View author publications You
can also search for this author inPubMed Google Scholar * Micheline Gourmelen View author publications You can also search for this author inPubMed Google Scholar ADDITIONAL INFORMATION This
work was supported by the Unité Propre de Recherche de l'Enseignement Supérieur Equipe d'Accueil 1531 (UPRES EA 1531 Paris 6 University) and by Unité Inserm U 515 Paris
Saint-Antoine. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Houang, M., Morineau, G., le Bouc, Y. _et al._ The Cortisol-Cortisone Shuttle in Children
Born with Intrauterine Growth Retardation. _Pediatr Res_ 46, 189–193 (1999). https://doi.org/10.1203/00006450-199908000-00011 Download citation * Received: 13 August 1998 * Accepted: 06
April 1999 * Issue Date: 01 August 1999 * DOI: https://doi.org/10.1203/00006450-199908000-00011 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content:
Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative







