- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT We wished to determine when each of the four NADPH oxidase components p22 phagocytic oxidase (phox), gp91 phox, p47 phox, p67 phox is first expressed embryologically and whether the
expression pattern occurs in a consistent temporal sequence or whether the four genes are expressed simultaneously. A deficiency of any one of them results in chronic granulomatous disease
(CGD). mRNA transcripts and protein expression for p22 phox, gp91 phox, p47 phox, p67 phox was monitored in murine embryos at time of implantation (E5.5) until E 11.5, and in fetal liver,
spleen, and limb bone marrow from E 14 until term (E 19). We observed that mRNA was first expressed for p22 phox at E 5.5, for p67 phox at E 7.0 and for p47 phox at E 7.5 before the onset of
yolk sac hematopoiesis (E 8.0). gp91 Phox mRNA was first expressed at E 9.0. However, only p22 phox protein was expressed in circulating hemacytoblast by E 9.0. No other embryonic tissue
contained phox proteins either before or after the establishment of hemocytoblastic circulation. The four specific mRNA transcripts and phox proteins were expressed in nests of developing
granulocytes in liver by E 14 and the expression continued in the liver at E 16 and E 19. Spleen and limb bone marrow showed inconsistent results. Cord blood neutrophils contained all phox
proteins. These studies confirm that the four CGD-related phox mRNA components of NADPH oxidase are expressed early in embryonic development and the expression occurs in a consistent
sequential fashion but only p22 phox protein appears in embryonic hemocytoblast. SIMILAR CONTENT BEING VIEWED BY OTHERS DIFFERENTIAL REGULATION OF FETAL BONE MARROW AND LIVER HEMATOPOIESIS
BY YOLK-SAC-DERIVED MYELOID CELLS Article Open access 14 May 2025 ROBUST TEMPORAL MAP OF HUMAN IN VITRO MYELOPOIESIS USING SINGLE-CELL GENOMICS Article Open access 24 May 2022
ONTOGENETICALLY DISTINCT NEUTROPHILS DIFFER IN FUNCTION AND TRANSCRIPTIONAL PROFILE IN ZEBRAFISH Article Open access 15 August 2023 MAIN We sought to determine the developmental expression
of NADPH oxidase components in mouse embryos. In humans, failure to express any one of four components results in a heterogenous group of inherited disorders of impaired superoxide
production in phagocytes termed CGD(1,2). At least five protein components have been characterized at a molecular level in both human(3–7) and mouse phagocytes(8–11), and the gene products
are designated phox, indicating phagocytic oxidase, p22 Phox and gp91 phox form a transmembrane alpha/beta complex known as cytochrome b(12,13) that associates with three cytosolic
components, p 47 phox, p 67 phox, and p 40 phox during activation of NADPH oxidase. Rac GTP-binding proteins are also involved(14). However, only gp91 phox, p22 phox, p47 phox, and p67 phox
gene mutations have been described as causes of CGD. We wished to determine the expression pattern of these four phox components of NADPH oxidase during the entire period of embryologic
development. We examined mouse embryos from postimplantation (E 5.5 d) through term (E 19 d) for expression of specific RNA transcripts of the four CGD-associated phox genes. In addition,
the presence or absence of specific phox protein was determined at the time of and after the onset of specific gene expression. MATERIALS AND METHOD RNA was prepared from whole mouse embryo
stages E 5.5, 6.0, 6.5, 7.0, 8.0, 9.0, 10.0, 10.5, 11.5. In addition, RNA was prepared from liver, spleen, and limbs of mid- and term fetuses (stage E 14, 16, 19). Reverse transcriptase PCR
was used using primer made to known nucleotide sequences of murine gp91 phox, p22 phox, p47 phox, and p67 phox genes to identify specific transcripts in mouse embryos and fetal liver,
spleen, and limbs. The PCR products for all phox genes were confirmed by DNA sequencing. Primers selected for identification of p22 phox were: upper primer 14 mer starting at nucleotide
position 89 of exon 2,5′-GGGGCATCGTGGCT-3′, lower primer 14 mer starting at nucleotide position, 431 of exon 3,5′-GGGGTGGGGGGTTG-3′. Optimal annealing temperature 48°C with 30 cycles yielded
a product length of 404 base pairs. Primers selected for identification of gp91 phox were: upper primer 16 mer starting at nucleotide position 986 murine cDNA 5′-GCCCCAAGGTATCCAA-3′, lower
primer 17 mer starting at nucleotide position 1524, murine cDNA 5′-GCCGTCCATACAGAGTC-3′. Optimal annealing temperature 50°C with 30 cycles yielded a product length of 555 base pairs. Primers
selected for identification of p47 phox were: upper primer 24 mer starting at exon 6,5′-ACATCACAGGCCCCATCATCCTTC-3′, lower primer 20 mer at exon 7,5′-ACCCACCTCGCTTTGTCTTC-3′. Optimal
annealing temperature 60°C with 35 cycles yielded a product length of 162 base pairs. Primers selected for identification of p67 phox were: upper primer 24 mer starting at exon
10,5′-TGGACTTCGGATTCACCCTCAGTC-3′, lower primer 24 mer starting at exon 135′-CACCTTGAGCATGTAAGGCATAGG-3′ Optimal annealing temperature 50°C with 30 cycles yielded a product length of 205
base pairs. The four primer sets were equally sensitive and detected mRNA in E 19 liver samples diluted 1:100 in E 5.0 which did not contain detectable mRNA for any of the phox genes. Serial
sections of embryos E 5.5, 6.5, 8.0, 9.0, 10.0 and sections of liver, spleen, and limbs containing bone marrow from fetuses stage E 14, 16, 19 were prepared for immunoperoxidase staining
using Zymed Histostain-SP and rabbit polyclonal anti-p22 phox antibody (gift from Dr. Mary Dinauer) and rabbit polyclonal anti-gp91 phox, anti-p47 phox, anti-gp40 phox and anti-p67 phox
(gift from Dr. John Curnutte, Genetech, San Francisco, CA). The presence of tissue and umbilical cord blood neutrophils was determined histologically employing rabbit polyclonal
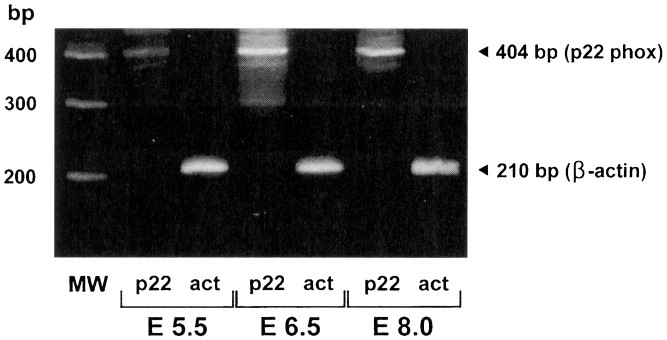
anti-lactoferrin and anti-muramidase (Zymed Labs. Inc., South San Francisco, CA 94080). RESULTS Transcripts to 22 kD phox were consistently identified in whole embryos starting at E 5.5
(Fig. 1). The onset of yolk sac hematopoiesis commences about stage E 8.0 to 8.5 in murine embryos(15). In contrast, transcripts to gp91 kD phox were not found in embryo stages E 5.5, 6.5,
8.0, 8.5, but were found in stages E 9 and all later stage embryos tested (up to E 11.5) (Fig. 2). Transcripts to p22 phox were identified in liver, limbs, and spleen at fetal stages E 14,
E16, and E19 and transcripts to gp91 phox were identified in liver but usually not in spleen and marrow at stages E 14, E 16, and E 19 (Fig. 3). Expression of p47 phox transcripts was not
detected in embryos at stages E 6.0 and E 7.0 but was found in all embryos tested between stages E 7.5 and E 11.5 (Fig. 4). In contrast, p67 phox transcripts were expressed in all embryos
between stages E 7.0 and E 11.5 but were not present at stage E 6.0 (Fig. 5). Transcripts to p47 phox and p67 phox were consistently expressed in fetal liver at stages E 14, E 16, and E 19.
Spleen and marrow inconsistently expressed modest levels of transcripts specific for p47 phox and p67 phox at all three stages of fetal organ development (data not shown). Immunoperoxidase
histochemistry of umbilical blood neutrophils from newborn mice identified each of the phox proteins of the NADPH oxidase (Fig. 6). Clusters of cells in the liver of E 14, 16, and 19 fetuses
were identified as neutrophils by positive staining for muramidase and lactoferrin. Similar clusters of cells also were positive for gp91 phox, p22 phox, p47 phox, p40 phox, and p 67 phox.
A positive pattern is shown for p22 phox and for granule lactoferrin and muramidase in Figure 7. E 14, E 16, and E 19 spleen and limb marrow did not contain identifiable cells containing
phox proteins, lactoferrin, or muramidase. No specific phox protein was identified in embryos before onset of vascularization and hematopoiesis, which occurs at approximately stage E 8. In
particular, despite the early expression of p22 phox transcripts at E 5.5, p22 phox was only identified in circulating hemocytoblasts at E 8.0, E 9.0, and E 10.0 (Fig. 8). These mononuclear
cells were negative for lactoferrin and muramidase. No other specific phox proteins were identified in circulating hemocytoblasts examined at E 8.0, E 9.0, and E 10.0. DISCUSSION NAPDH
oxidase is a unique complex of proteins highly expressed in blood phagocytes of all mammalian species and is responsible for the respiratory burst required for normal killing of bacteria and
fungi(1,2). The complex consists of a 91-kD glycoprotein (gp91 phox) and a 22-kD protein (p22 phox) that form an alpha-beta dimer constitutively expressed in plasma membranes and specific
granule membranes of human and murine blood neutrophils(16,17). CGD results from mutations in either gp91 phox, p22 phox, p47 phox, or p67 phox genes. The genes for phox proteins have been
mapped and sequenced in both humans(3,4,10,18) and mice(7–9,11). We wished to study the developmental expression of the four CGD-related components of the murine NADPH oxidase by employing
an _in vivo_ embryonic mouse system that monitors the time of onset of gene expression and the localization of expressed protein. Embryonic and fetal hematopoiesis has been well
characterized in the mouse(15,19). The phenomenon is first extraembryonic, occurring in blood islands in the yolk sac at stage E 8. The precursors of these islands known as hemangioblasts
migrate from the primitive streak region of blastoderm by invaginating through the primitive streak spreading laterally to produce the mesodermal layer. Hemangioblasts differentiate in two
directions, one forming angioblasts more peripherally and the other forming hemoblasts centrally. Gross and microscopic evidence of an embryonic red blush due to circulating nucleated
hemocytoblasts was observed by us between E 8 and E 9. Liver hemopoiesis is detectable in the mouse by d 10 (E 10). The liver then remains the major hemopoietic organ during fetal life.
Splenic hemopoiesis is initiated by about d 15(E 15) and continues throughout fetal life. The bone marrow is the final hemopoietic site during fetal development with hemopoiesis commencing
between E 17 and E 18. The early expression of transcription factors GATA-1, GATA-3, and vav are required for initiation of hemopoiesis(20–23). The initial development of hemocytoblasts
occurs independent of bone marrow growth factors but by E 10 differentiation along either erythroid or granulocyte-macrophage lineage does require specific growth factors. Although we found
p22 phox protein in the circulating hemocytoblasts of E 8.0, E 9.0, and E 10.0, the cells were negative as expected for lactoferrin and muramidase, two markers of differentiated myeloid
cells(24). These hemocytoblasts were also negative for the other phox proteins to the limits of detection as determined by standard immunoperoxidase histochemistry although RNA transcripts
for p47 phox and p67 phox were found in embryos at these stages of development. The detection of p22 phox without the other components is also consistent with the previous observation that
its expression, unlike the other oxidase genes, is not lineage specific(13). Transcripts for all CGD-associated NADPH oxidase components were found in the liver of fetuses at E 14, E 16, and
E 19. Nests of cells strongly positive for lactoferrin and muramidase confirmed the presence of granulopoiesis in fetal liver at each of these stages. Similar nests of cells in the liver of
E 14, E 16, and E 19 also were strongly positive for gp91 phox, p47 phox, and p67 phox proteins. Spleens and limb marrow from E 14, E 16, and E 19 did not contain cells with identifiable
phox proteins which may reflect the delay in onset of granulopoiesis in these fetal tissues and the predominance of liver as the primary site of granulopoiesis in the fetal mouse. The
expression of RNA transcripts of p22 phox in embryos as early as E 5.5 remains unexplained. It was not associated with any identifiable p22 phox protein in serial whole mount sections
through the entire embryo. Since gp91 phox transcripts and protein were not found in these early stage embryos, the lack of p22 phox could be explained by the known requirement of gp91 phox
for the stabilization of p22 phox in cells expressing these proteins(12,17,25). Furthermore, the expression of transcripts for p47 phox and p67 phox also occurred before the onset of
hemopoiesis (E 7.0 and E 7.5, respectively) but again, serial sections of whole mount embryos failed to reveal either p47 phox or p67 phox protein. Finally, even when gp91 phox transcripts
were expressed at E 9.0, no gp91 phox protein was found in hemocytoblasts expressing p22 phox protein within the sensitivity limits of our assay. The _in vivo_ temporal sequential pattern
expression of mRNA and proteins required for activation of a complex biologic system has been studied previously. The major cytoskeletal proteins α-spectrin, β-spectrin, and ankyrin are
synthesized and assembled into a supportive membrane skeleton during murine erythroid differentiation initially in embryonic yolk sac hemoblast and later in fetal liver cells(26).
Disaggregated embryonic yolk sac cells and circulating blood cells from E 9 embryos before formation of fetal liver are capable of colonizing E 11 to E 15 fetal liver to initiate hemopoiesis
supporting the notion that embryonic shifts in hemopoietic sites occurs via the fetal circulation(27). We observed a consistent temporal sequential pattern expression for the four
CGD-related NADPH oxidase components during murine embryonic and fetal development rather than an abrupt synchronous expression of all components at one stage of development. p22 Phox mRNA
is first expressed at E 5.5, the expression of p47 phox mRNA occurs at E 7.0 and the expression of p67 phox mRNA occurs at E 7.5. All stages are before the onset of hemopoiesis whereas gp91
phox mRNA first occurs at E 9.0. However, none of the phox proteins (including p40 phox and myeloid markers lactoferrin and muramidase) except p22 phox were found in circulating
hemocytoblasts. The fetal liver at stages E 14, E 16, and E 19 contains nests of cells that contain lactoferrin, muramidase, and all phox proteins indicating full maturity of the NADPH
oxidase in fetal liver myeloid cells. Indeed, umbilical cord blood neutrophils of newborn mice also contain all phox proteins. This observation is consistent with results of fetal human
granulocytes and prenatal studies on human fetuses evaluated for CGD. Both studies reported normal functional phagocytic oxidase activity of fetal blood neutrophils including two potentially
affected males(28,29). ABBREVIATIONS * CGD: chronic granulomatous disease * E+ number: refers to specific embryonic stage of development from day of fertilization * phox: phagocytic oxidase
REFERENCES * Smith RM, Curnutte JT 1991 Molecular basis of chronic granulomatous disease. _Blood_ 77: 673–686 Article CAS Google Scholar * Dinauer MC 1993 The respiratory burst oxidase
and the molecular genetics of chronic granulomatous disease. _Crit Rev Clin Lab Sci_ 30: 329–369 Article CAS Google Scholar * Royer-Pokora B, Kunkel LM, Monaco P, Goff SC, Newberger PE,
Baehner, RL, Cole FS, Curnutte, JT, Orkin SH 1986 Cloning the gene for an inherited human disorder-chronic granulomatous disease-on the basis of its chromosomal location. _Nature_ 322: 32–37
Article CAS Google Scholar * Dinauer, MC, Pierce EA, Bruns GA, Curnutte JT, Orkin SH 1990 Human neutrophil cytochrome b light chain (p22-phox). _J Clin Invest_ 86: 1729–1737 Article CAS
Google Scholar * Volpp BD, Nauseef WM, Donelson JE, Moser DR, Clark RA 1990 Cloning of the cDNA and functional expression of the 47-kilodalton cytosolic component of human neutrophil
respiratory burst oxidase. _Proc Natl Acad Sci USA_ 86: 7195–7197 Article Google Scholar * Leto TL, Lomax KL, Volpp BD, Nunoi H, Sechler JM, Nauseef WM, Clark RA, Gallin JI, Malech HL 1990
Cloning of a 67-kd neutrophil oxidase factor with similarity to a noncatalytic region of p60-src. _Science_ 248: 727–730 Article CAS Google Scholar * Zhan SZ, Vazquez N, Zhan S, Wientjes
FB, Budarf ML, Schrock E, Reid T, Green ED, Chanock SJ 1996 Genomic structure, chromosomal localization, start of transcription, and tissue expression of the human p40-phox, a new component
of the nicotinamide adenine dinucleotide phosphate-oxidase complex. _Blood_ 88: 2714–2721 Article CAS Google Scholar * Bjorgvinsdottir H, Zhen L, Dinauer MC 1996 Cloning of murine gp91
phox cDNA and functional expression in a human X-linked chronic granulomatous disease. _Blood_ 87: 2005–2010 Article CAS Google Scholar * Jackson SH, Malech HL, Kozak, CA, Lomax KJ,
Gallin JI, Holland SM 1994 Cloning and functional expression of the mouse homologue of p47 phox. _Immunogenics_ 39: 272–275 CAS Google Scholar * Franke U, Hsieh CL, Follmer BE, Lamas K.,
Maleic HL, Leto TL 1990 Genes for two autosomal recessive forms of chronic granulomatous disease assigned to 1q25 (NCF2) and 7q11:23 (NCF1): _Am J Hum Genet_ 47: 483–492 Google Scholar *
Zhan S, Kozak CA, Chanock SJ 1997 Cloning and chromosomal localization of ncf4, the mouse homologue of p40-phox. _Immunogenetics_ 45: 217–219 Article CAS Google Scholar * Dinauer MC,
Orkin SH, Brown R, Jesaitis AJ, Parkos CA 1987 The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. _Nature_
327: 717–721 Article CAS Google Scholar * Parkos CA, Dinauer MC, Walker LE, Allen RA, Jesaitis AJ, Orkin SH 1988 Primary structure and unique expression of the 22-kilodalton light chain
of human neutrophil cytochrome b. _Proc Natl Acad Sci USA_ 85: 3319–3323 Article CAS Google Scholar * Bokoch GM 1994 Regulation of the human neutrophil NADPH oxidase by the rac
GTP-binding proteins. _Curr Opin Cell Biol_ 6: 212–218 Article CAS Google Scholar * Tavassoli M 1991 Embryonic and fetal hematopoiesis: an overview. _Blood Cells_ 1: 269–281 Google
Scholar * Parkos CA, Allen RA, Cochrane CG, Jesaitis AJ 1987 Purified cytochrome b from human plasma membrane is comprised of two polypeptides with relative molecular weights of 91:000 and
22,000. _J Clin Invest_ 80: 732–741 Article CAS Google Scholar * Segal AW 1987 Absence of both cytochrome b-245 subunits from neutrophils in X-linked chronic granulomatous disease.
_Nature_ 326: 88–91 Article CAS Google Scholar * Chanock SJ, Barrett DM, Curnutte JC, Orkin SH 1991 Gene structure of the cytosolic component, phox-47 and mutations in autosomal recessive
chronic granulomatous disease. _Blood_ 78: 165a Google Scholar * Moore MAS, Metcalf D 1970 Ontogeny of the hematopoietic system: yolk sac origin of in vivo and in vitro colony forming
cells in the developing mouse embryo. _Br J Haematol_ 18: 279–296 Article CAS Google Scholar * Sposi NM, Zon LI, Care A, Valtieri M, Testa U, Gabbianelli M, Mariani G, Bottero L, Mather
C, Orkin SH 1992 cycle-dependent initiation and lineage-dependent abrogation of GATA-1 expression in pure differentiating hematopoietic progenitors. _Proc Natl Acad Sci USA_ 89: 6353–6357
Article CAS Google Scholar * Keller G, Kennedy M, Papayannopoulou T, Wiles MV 1993 Hematopoietic commitment during embryonic stem cell differentiation in culture. _Mol Cell Biol_ 13:
473–486 Article CAS Google Scholar * Shivdasani RA, Mayer EL, Orkin SH 1995 Absence of blood formation in mice lacking the T- cell leukemia oncoprotein tal-1/SCL. _Nature_ 373: 432–434
Article CAS Google Scholar * Kallianpur, AR, Jordan JE, Brandt SJ 1994 The SCL/TAL-1 gene is expressed in progenitors of both the hematopoietic and vascular systems during embryogenesis.
_Blood_ 83: 1200–1208 Article CAS Google Scholar * Borregaard N, Kjeldsen L, Lollike K, Sengelov H 1995 Granules and secretory vesicles of the human neutrophil. _Clin Exp Immunol_ 101(
suppl 1): 6–9 Article Google Scholar * Parkos CA, Dinauer MC, Jesaitis AJ, Orkin SH, Curnutte JT 1989 Absence of both the 91-kD and 22-kD subunits of human cytochrome b in two genetic
forms of chronic granulomatous disease. _Blood_ 73: 1416–1420 Article CAS Google Scholar * Peters LL, White RA, Birkenmeier CS, Bloom ML, Lux SE, Barker, JE 1992 Changing patterns in
cytoskeletal mRNA expression and protein synthesis during murine erythropoiesis in vivo. _Proc Nat Acad Sci USA_ 89: 5749–5753 Article CAS Google Scholar * Toles JF, Chui DH, Belbeck LW,
Starr E, Barker JE 1989 Hemopoietic stem cells in murine embryonic yolk sac and peripheral blood. _Proc Soc Nat Acad Sci USA_ 86: 7456–7459 Article CAS Google Scholar * Newburger PE 1982
Superoxide generation by human fetal granulocytes. _Pediatr Res_ 16: 373–376 Article CAS Google Scholar * Matthay KK, Golbus MS, Wara DW, Mentzer WC 1984 Prenatal diagnosis of chronic
granulomatous disease. _Am J Med Genet_ 17: 731–739 Article CAS Google Scholar Download references ACKNOWLEDGEMENTS The authors thank Drs. Sharon H. Jackson and Steven M. Holland for
providing PCR primer pair information for mouse p47 phox and mouse p67 phox. We also thank Drs. Mary Dinauer and John Curnutte for providing antibodies to the mouse phox proteins. I am
grateful to Drs. Hal Slavkin and Charles Shuler for allowing me (RLB) to work in their laboratory. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pediatrics, School of Medicine,
School of Dentistry, University of Southern California, Los Angeles, California Robert L Baehner * Center for Craniofacial Molecular Biology, School of Dentistry, University of Southern
California, Los Angeles, California Sharon Millar-Groff & Pablo Bringas Authors * Robert L Baehner View author publications You can also search for this author inPubMed Google Scholar *
Sharon Millar-Groff View author publications You can also search for this author inPubMed Google Scholar * Pablo Bringas View author publications You can also search for this author inPubMed
Google Scholar ADDITIONAL INFORMATION Supported in part by CCMB National Institutes of Health Center Grant NIDR P50DE09166-10.Results presented in part at the Annual Meetings of the
American Society of Hematology, December 1995 and 1996, Seattle WA and Orlando FL. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Baehner, R.,
Millar-Groff, S. & Bringas, P. Developmental Expression of NADPH Phagocytic Oxidase Components in Mouse Embryos. _Pediatr Res_ 46, 152–157 (1999).
https://doi.org/10.1203/00006450-199908000-00004 Download citation * Received: 02 October 1998 * Accepted: 26 April 1999 * Issue Date: 01 August 1999 * DOI:
https://doi.org/10.1203/00006450-199908000-00004 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative









