- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Antenatal glucocorticoid therapy improves pulmonary function in preterm newborns. We have determined the effect of antenatal glucocorticoid therapy on isoproterenol and
prostaglandin (PG) E2-mediated relaxation in preterm ovine pulmonary veins after birth. Ovine fetuses (121 and 126 d of gestation; term = 150 d) received an ultrasound guided intramuscular
injection of betamethasone, 0.5 mg/kg, or saline. Lambs were delivered 15 or 48 h later, ventilated for 3 h, and killed. Isolated fourth generation pulmonary veins were suspended in organ
chambers filled with modified Krebs-Ringer solution(95% O2, 5% CO2) at 37 °C, and their isometric tension was recorded. During contractions to U46619, isoproterenol and PGE2 induced greater
relaxations of pulmonary veins of betamethasone-treated lambs than those of control. Forskolin, an activator of adenylate cyclase, caused greater relaxation in veins of betamethasone-treated
lambs than in those of controls. A greater relaxation of veins treated with betamethasone than that of control veins also occurred in the presence of isobutylmethylxanthine, an inhibitor of
phosphodiesterases. All vessels relaxed similarly to 8-bromo-cAMP, a cell membrane-permeable analog of cAMP. When stimulated with isoproterenol, PGE2, and forskolin, adenylate cyclase
activity of crude membrane preparations of pulmonary veins treated with betamethasone was greater than that of controls. These results demonstrate that antenatal betamethasone therapy
potentiates isoproterenol and PGE2-mediated relaxation of pulmonary veins of preterm lambs; an enhanced adenylate cyclase activity explain in part the effect of antenatal glucocorticoid
therapy on pulmonary veins of preterm lambs. SIMILAR CONTENT BEING VIEWED BY OTHERS POSTNATAL BUDESONIDE IMPROVED LUNG FUNCTION IN PRETERM LAMBS EXPOSED TO ANTENATAL STEROIDS AND
CHORIOAMNIONITIS Article 17 February 2024 ANTENATAL CORTICOSTEROIDS: A REAPPRAISAL OF THE DRUG FORMULATION AND DOSE Article Open access 11 November 2020 VARIABLE EFFECT OF THE POST-PARTUM
MENSTRUAL CYCLE ON ALDOSTERONE AND RENIN IN WOMEN WITH RECENT PREECLAMPSIA Article 19 June 2024 MAIN Antenatal glucocorticoid treatment improves pulmonary function in the preterm infant
after birth(1–4). We have shown that a single dose of antenatal betamethasone augments nitric oxide-mediated relaxation of pulmonary veins of preterm lambs(5). Under a variety of conditions,
pulmonary veins exhibit marked vasoactivity and contribute to a significant part of pulmonary vascular resistance(6–11). A decrease in venous resistance may help to reduce pulmonary
vascular resistance and pulmonary edema after birth(6, 8–10). In the fetus and newborn, β-adrenergic agonists and PGE2 are important vasodilators of pulmonary vessels(12, 13). They activate
adenylate cyclase of vascular smooth muscle cells, which causes an increase in intracellular content of cAMP and consequently causes vasodilation(13, 14). In cultured smooth muscle cells of
rat mesenteric arteries, incubation with dexamethasone enhances adenylate cyclase activity(15). If this is also true under _in vivo_ conditions, antenatal glucocorticoid therapy may
potentiate action of β-adrenergic agonists and PGE2-mediated effects on pulmonary vessels of preterm lambs. The present study was designed to determine the effect of antenatal glucocorticoid
therapy on the response of isolated pulmonary veins of preterm lambs to the β-adrenergic agonist isoproterenol and PGE2. METHODS _ANIMALS_. Twenty-two pregnant ewes carrying singleton
fetuses(Nebeker Ranch, Palmdale, CA) were used for the study. At 121 or 126 d of gestation (term = 150 d), the fetus was injected intramuscularly in either the neck or shoulder (using a
9-cm, 20-gauge spinal needle) with betamethasone 0.5 mg/kg body weight (Celestone Soluspan®, Schering Pharmaceuticals, Kenilworth, NJ; _n_ = 11) or 0.15 M saline (_n_ = 11). Fetal injection
procedures were performed with the ewe in a shearing position, held from behind by a seated assistant. A real time ultrasound system (Acusonic Opus I, Lane Cove, Australia) and a 5-mHZ
sector transducer were used to image the fetus and guide the intramuscular injection. The body weight of the fetus was estimated based on earlier studies; the injection volume was 2.5 mL,
and the betamethasone dose was based on previously demonstrated improvements in pulmonary function of premature ovine lambs(3). The injection procedure, including positioning of the ewe,
generally took <5 min. The procedure does not acutely alter the fetal catecholamine level(16). Animal handling and study protocols were reviewed and approved by the Harbor-UCLA Animal
Care and Use Review Committee. Fifteen hours later for 121-d gestation lambs and 48 h later for 126-d gestation lambs, the lambs were delivered by cesarean section, treated with surfactant
(Survanta, Ross Laboratories, Columbus, OH; 100 mg/kg) by direct intratracheal instillation, and then mechanically ventilated with 100% O2 for 3 h. It is necessary to mechanically ventilate
these lambs as spontaneously breathing preterm lambs at this gestation will not survive. We were interested in studying the function of the pulmonary vasculature after it had undergone the
postnatal transition. During this period, data were gathered to determine the effect of antenatal betamethasone therapy on a variety of physiologic responses(4). After 3 h of ventilation,
the lambs were killed with an overdose of pentobarbital (100 mg/kg, i.v.), and the lungs were removed. Fourth generation pulmonary veins were dissected from the lungs and cut into rings
(length, 3 mm; outside diameter, 1.5-2.0 mm). _ORGAN CHAMBER STUDY_. Rings of isolated fourth generation pulmonary veins were suspended in organ chambers filled with 10 mL of modified
Krebs-Ringer bicarbonate solution [composition (in mM): NaCl, 118.3; KCl, 4.7; CaCl2, 2.5; MgSO4, 1.2; KH2PO4, 1.2; NaHCO3, 25.0; glucose, 11.1] maintained at 37 ± 0.5 °C and aerated with
95% O2-5% CO2 (pH = 7.4). Each ring was suspended by two stirrups passed through the lumen. One stirrup was anchored to the bottom of the organ chamber; the other one connected to a
force-displacement transducer (model FT03C, Grass Instrument Co., Quincy, MA) for the measurement of isometric force(5, 17). At the beginning of each experiment, vessel rings were brought to
their optimal tension by stretching the tissues progressively until the contractile responses to 100 mM KCl were maximal. There was no significant difference in optimal resting tension
between pulmonary veins from control and betamethasone-treated lambs (0.31 ± 0.03 g _versus_ 0.29± 0.04 g; _n_ = 11 for each group, _p_ > 0.05). Relaxation of pulmonary veins to different
agents was determined during contraction to U46619 (3 × 10-8 M; a thromboxane A2 analog)(18). Relaxations induced by isoproterenol, PGE2, and 8-bromo-cAMP were tested in veins of 126-d
gestation; relaxation induced by forskolin was tested in both 121- and 126-d gestation lambs. The response induced by forskolin was not significantly different between veins of 121-d and
those of 126-d gestation lambs (see Figs. 3 and 4). To obtain concentration-response curves, these vasodilators were administrated in a cumulative fashion. For each vessel ring, only one
vasodilator was tested. To exclude the involvements of cyclooxygenase products and endothelium-derived nitric oxide(5, 11, 19, 20), all vessels were treated with indomethacin (10-5 M) plus
nitro-L-arginine (10-4 M) before they were contracted with U46619. Indomethacin plus nitro-L-arginine raised the basal tension of pulmonary veins of control and betamethasone by 0.45 ± 0.07
g (_n_ = 22) and 0.39 ± 0.10 g (_n_= 22), respectively. These values were not significantly different(_p_ > 0.05). _ADENYLATE CYCLASE ASSAY_. Pulmonary veins were homogenized in 10
volumes of ice-cold Tris/HCl buffer (50 mM, pH 7.6) containing DTT (1 mM), EDTA (0.5 mM), IBMX (1 mM), and phenymethylsulfonyl fluoride (1 mM). The homogenate was then filtered through two
layers of gauze, and adenylate cyclase activity was determined on the resultant crude membrane preparation by a modification of the method of Shaul _et al._(22). Further purification of the
membrane was not performed because isoproterenol-sensitive adenylate cyclase activity decreases with subsequent steps of smooth muscle membrane preparation(21, 22). The protein
concentrations of the homogenate were determined by Bradford(23) method using BSA as the standard. Pulmonary vessel membranes (20 μg of protein) were incubated at 30°C for 10 min in 150 μL
of HEPES buffer [composition: HEPES (50 mM), Tris-HCl (10 mM), DTT (0.1 mM), ATP (1 mM), MgCl2 (10 mM), creatine phosphate (12 mM), creatine phosphokinase (185 U/mL), ascorbic acid (0.5 mM),
and IBMX (1 mM)] under control conditions or in the presence of isoproterenol(10-6 M), PGE2 (3 × 10-7 M), or forskolin (3× 10-6 M). Preliminary experiments confirmed the linearity of
adenylate cyclase activity at the protein concentrations up to 40 μg and incubation times up to 20 min. The reaction was terminated by placing the assay tubes in a boiling water bath for 5
min. The samples were then centrifuged at 10 000 × _g_ for 15 min. The supernatant was extracted with 4 volumes of water-saturated diethyl ether and lyophilized. The lyophilized samples were
resuspended in 0.5 mL of sodium acetate buffer (0.05 M, pH 6.2), and the content of cAMP was measured using a cAMP RIA kit(Biomedical Technologies Inc., Stoughton, MA). The amount of cAMP
produced during the incubation was calculated by subtracting the cAMP content of blanks in which the adenylate cyclase activity was inhibited before the onset of incubation, yielding
adenylate cyclase activity expressed as picomoles of cAMP/mg of protein/min. _DRUGS_. The following drugs were used (unless otherwise specified, all were obtained from Sigma Chemical Co.,
St. Louis, MO): 8-bromo-adenosine 3′5′-cyclic monophosphate (8-bromo-cAMP), forskolin, indomethacin, IBMX, isoproterenol bitartrate,_N_G-nitro-L-arginine (Research Biochemicals
International, Natick, MA), phentolamine mesylate (Research Biochemicals International), PGE2, and U46619(9,11-dideoxy-11α,9α-epoxymethanoprostaglandin PGF2α). IBMX was dissolved in ethanol
(final concentration in an organ chamber, 0.1%). Preliminary experiments indicated that ethanol at this concentration did not significantly affect contraction of the tissues to U46619 nor
the relaxation to isoproterenol and PGE2 (data not shown). Indomethacin(10-5 M) was prepared in equimolar Na2CO3. This concentration of Na2CO3 did not significantly affect the pH of the
solution in the organ chamber. The other drugs were prepared using distilled water. _DATA ANALYSES_. Contractions are expressed as gram tension developed. Relaxations are expressed as
percent of contraction of tissues to U46619. Data are shown as means ± SEM. When data from two groups of vessels were compared, a _t_ test for unpaired observations was used. When data from
the same group of vessels, before and after stimulation, were compared, a _t_ test for paired observations was used. Comparison of mean values of more than two groups was made with a one-way
ANOVA test with Student-Newman-Keuls test for _post hoc_ testing of multiple comparison. All analyses were performed using a commercially available statistics package (SigmaStat, Jandel
Scientific, San Rafael, CA). Statistical significance was accepted when the _p_ value (two-tailed) was less than 0.05. In all experiments, _n_ represents the number of veins from different
lambs. RESULTS _ORGAN CHAMBER STUDIES_. Relaxation of isolated pulmonary veins was determined after their tensions were raised with U46619 (3 × 10-8 M). There was no significant difference
in the increases in tension elicited with U46619 between veins from control and those from betamethasone-treated lambs (1.83 ± 0.22 g _versus_ 1.76± 0.25 g; _n_ = 11 for each group, _p_ >
0.05). During contraction, isoproterenol and PGE2 caused concentration-dependent relaxations of pulmonary veins with threshold concentrations at 10-9 M and 3 × 10-9 M, respectively.
Relaxations induced by these agents were significantly greater in veins of betamethasone-treated lambs than in those of controls (Figs. 1 and 2). Forskolin, an activator of adenylate
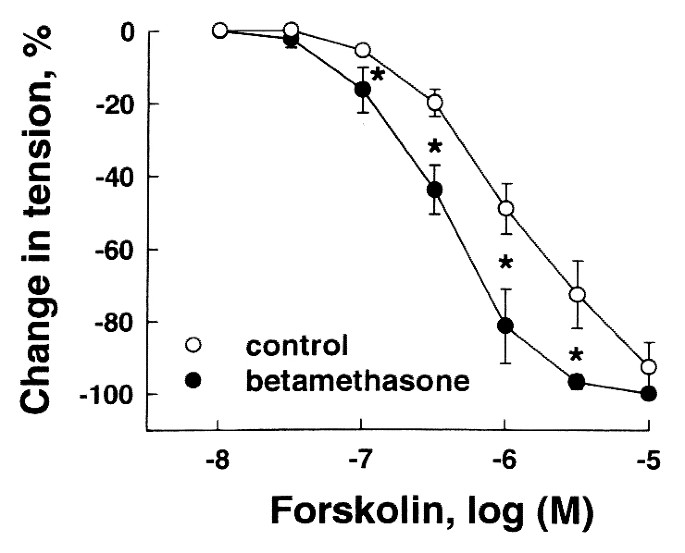
cyclase(24), also caused greater relaxation of pulmonary veins of betamethasone-treated lambs than those of controls (Fig. 3). In the presence of IBMX (10-5 M), an inhibitor of
phosphodiesterases(25), forskolin-induced relaxation of all vessels was augmented; however, relaxation of veins treated with betamethasone was significantly greater than of control veins
(Fig. 4). When stimulated with 8-bromo-cAMP, a cell membrane permeable analog of cAMP(26), pulmonary veins of control lambs relaxed to a similar extent as those from betamethasone-treated
lambs (Fig. 5). _ADENYLATE CYCLASE ACTIVITY ASSAY_. The basal activity of adenylate cyclase of pulmonary veins of control and betamethasone-treated lambs was 4.99 ± 0.69 pmol of cAMP/mg of
protein/min (_n_ = 7) and 7.921 ± 0.80 pmol of cAMP/mg of protein/min (_n_ = 7), respectively. These values were significantly different (_p_ < 0.05). When stimulated with isoproterenol
(10-6 M), PGE2 (3× 10-7 M), and forskolin (3 × 10-6 M), the activities of adenylate cyclase of pulmonary venous preparations treated with betamethasone were significantly greater than those
of control (Fig. 6). DISCUSSION The present study demonstrated that a single dose of antenatal betamethasone significantly augmented relaxation of pulmonary veins toβ-adrenergic agonist
isoproterenol and PGE2. The augmented responses to these vasodilators were observed at concentrations as low as 10-9 M to 3 × 10-9 M. Thus, the effect of betamethasone seems to occur within
the physiologic range of concentration ofβ-adrenergic agonist and PGE2(27–30). Isoproterenol and PGE2 cause vasodilation by activating adenylate cyclase and subsequently increasing the
intracellular content of cAMP in the smooth muscle cell(13, 14, 31). In the present study, forskolin, an activator of adenylate cyclase(24), also caused greater relaxation of pulmonary veins
of betamethasone-treated lambs than of control. Moreover, under basal conditions and after isoproterenol, PGE2, and forskolin, adenylate cyclase activity of the crude membrane preparation
of pulmonary veins treated with betamethasone was greater than that of control. Thus, an enhanced adenylate cyclase activity was at least partially responsible for the augmented relaxation
of pulmonary veins of preterm lambs after betamethasone treatment. These results are consistent with the report that, in cultured smooth muscle cells of rat mesenteric arteries, 24- and 48-h
incubation with dexamethasone enhances adenylate cyclase activity(15). We found that isoproterenol and PGE2, at concentrations inducing comparable relaxations of veins as that of forskolin,
caused a smaller increase in adenylate cyclase activity than did forskolin. Such a phenomenon has been observed in both pulmonary and nonpulmonary smooth muscles by other investigators. The
underlying mechanism is not clear(21, 22). cAMP is degraded by phosphodiesterases(25). Therefore, it is no surprise that IBMX, an inhibitor of phosphodiesterases(25), augmented relaxation
of all vessels to forskolin. In the presence of IBMX, forskolin still caused greater relaxation of pulmonary veins from betamethasone-treated lambs than those from control lambs. Because the
inhibition of phosphodiesterases with IBMX did not reduce the difference in forskolin-induced relaxation between control and betamethasone-treated veins, phosphodiesterases seem unlikely to
contribute significantly to the effect of betamethasone treatment on pulmonary veins of preterm lambs. The basal activity of adenylate cyclase activity was greater in betamethasone-treated
veins than in the control. This would result in a greater basal content of cAMP in betamethasone-treated veins and thus we expected that the treated veins could show a greater relaxation to
8-bromo-cAMP (a cell membrane-permeable analog of cAMP)(26) than control veins. However, all vessels relaxed similarly to those treated with 8-bromo-cAMP. Because 8-bromo-cAMP does not
stimulate adenylate cyclase activity, the difference in basal activity of adenylate cyclase between control and treated veins may not be large enough to make betamethasone-treated veins
relax significantly more to the cAMP analog than do control veins. However, when adenylate cyclase is stimulated by isoproterenol, PGE2, and forskolin, the difference in the activity of
adenylate cyclase between control and betamethasone groups resulted in a greater relaxation of treated veins to these agents. Pulmonary vascular resistance is regulated not only by
vasoactivity of pulmonary arteries but also the vasoactivity of pulmonary veins(6). There is a body of literature showing that pulmonary veins exhibit significant vasoactivity in response to
a variety of vasoactive mediators(6–11). In the present study, we demonstrated that antenatal glucocorticoid therapy augments relaxation of pulmonary veins of preterm lambs to a
β-adrenergic agonist and PGE2. In premature human infants, antenatal glucocorticoid therapy improves pulmonary function and reduces the incidences of respiratory distress syndrome(1, 2). If
our present results are applicable to humans _in vivo,_ an augmentation of adenylate cyclase-cAMP-mediated response of pulmonary veins after glucocorticoids may help to reduce pulmonary
vascular resistance and pulmonary edema after birth. ABBREVIATIONS * PG: prostaglandin * IBMX: isobutylmethylxanthine REFERENCES * Doyle LW, Kitchen WH, Ford GW, Richards AL, Lissenden JV,
Ryan MM 1986 Effects of antenatal steroid therapy on mortality and morbidity in very low birth weight infants. _J Pediatr_ 108: 287–292. Article CAS PubMed Google Scholar * Crowley P,
Chalmers I, Keirse MJ 1990 The effects of corticoid administration before preterm delivery: overview of the evidence from controlled trials. _Br J Obstet Gynaecol_ 97: 11–25. CAS PubMed
Google Scholar * Jobe AH, Polk DH, Ikegami M, Sly P, Kohen R, Kelly R 1993 Lung responses to ultrasound-guide fetal treatments with corticoids in preterm lambs. _J Appl Physiol_ 75:
2099–2105. Article CAS PubMed Google Scholar * Chen C-M, Ikegami M, Ueda T, Polk DH, Jobe AH 1995 Fetal corticoid and T4 treatment effects on lung function of surfactant-treated preterm
lambs. _Am J Respir Crit Care Med_ 151: 21–26. Article CAS PubMed Google Scholar * Zhou H, Gao Y, Raj JU 1996 Antenatal betamethasone therapy augments nitric oxide-mediated relaxation of
preterm ovine pulmonary veins. _J Appl Physiol_ 80: 390–396. Article CAS PubMed Google Scholar * Raj JU, Chen P 1986 Microvascular pressures measured by micropuncture in isolated lamb
lungs. _J Appl Physiol_ 61: 2194–2201. Article CAS PubMed Google Scholar * Raj JU, Anderson J 1990 Pulmonary venous responses to thromboxane A2 analogue and atrial natriuretic peptide in
lambs. _Circ Res_ 66: 492–502. Article Google Scholar * Raj JU, Hillyard R, Kaapa P, Gropper M, Anderson J 1990 Pulmonary arterial and venous constriction during hypoxia in 3- to 5-wk-old
and adult ferrets. _J Appl Physiol_ 69: 2183–2189. Article CAS PubMed Google Scholar * Pritze S, Simmet T, Peskar BA 1991 Effect of platelet-activating factor on porcine pulmonary blood
vessels _in vitro_. _Naunyn-Schmiedebergs Arch Pharmacol_ 344: 495–499. Article CAS PubMed Google Scholar * Zhao Y, Packer CS, Rhoades RA 1993 Pulmonary vein contracts in response to
hypoxia. _Am J Physiol_ 265:L87–L92. CAS PubMed Google Scholar * Gao Y, Zhou H, Raj JU 1995 PAF induces relaxation of pulmonary arteries but contraction of pulmonary veins in the ferret.
_Am J Physiol_ 269:H704–H709. CAS PubMed Google Scholar * Cassin S 1980 Role of prostaglandins and thromboxanes in the control of the pulmonary circulation in the fetus and newborn.
_Semin Perinatol_ 4: 101–107. CAS PubMed Google Scholar * Shaul PW, Farrar MA, Buja LM 1991 Ontogeny ofβ-adrenergic regulation of adenylate cyclase in intrapulmonary arteries from fetal
and postnatal lambs. _Pediatr Res_ 30: 610–615. Article CAS PubMed Google Scholar * Nijkamp FP, Engels F, Henricks PA, Van Oosterrhout JM 1992 Mechanisms of β-adrenergic receptor
regulation in lungs and its implications for physiological responses. _Physiol Rev_ 72: 323–367. Article CAS PubMed Google Scholar * McLellan AR, Tawil S, Lyall F, Milligan G, Connell
JMC, Kenyon CJ 1992 Effect of dexamethasone on G protein levels and adenylate cyclase activity in rat vascular smooth muscle cells. _J Mol Endocrinol_ 9: 237–244. Article CAS PubMed
Google Scholar * Newnham JP, Polk DH, Kelly RW, Padbury JF, Evan SF, Ikegami M, Jobe AH 1994 Catecholamine response to ultrasonographically guided percutaneous blood sampling in fetal
sheep. _Am J Obstet Gynecol_ 171: 460–465. Article CAS PubMed Google Scholar * Gao Y, Zhou H, Raj JU 1995 Heterogeneity in the role of endothelium-derived nitric oxide in pulmonary
arteries and veins of term fetal lambs. _Am J Physiol_ 268:H1586–H1592. Article CAS PubMed Google Scholar * Coleman RA, Humphrey PPA, Kennedy I, Levy GP, Lumley P 1981 Comparison of the
actions of U-46619, a prostaglandin H2-analogue, with those of prostaglandin H2 and thromboxane A2 on isolated smooth muscle preparations. _Br J Pharmacol_ 73: 773–778. Article CAS PubMed
PubMed Central Google Scholar * Vane J, Botting R 1987 Inflammation and the mechanism of action of antiinflammaroty drugs. _FASEB J_ 1: 89–96. Article CAS PubMed Google Scholar *
Mülsch A, Busse R 1990 _N_G-nitro-L-arginine(_N_G-[iminonitroamine)methyl]-L-ornithine) impairs endothelium-dependent dilations by inhibiting cytosolic nitric oxide synthesis from
L-arginine. _Naunyn-Schmiedebergs Arch Pharmacol_ 341: 143–147. PubMed Google Scholar * Popovich KJ, Hiller C, Hough A, Norris JS, Cornett LE 1984 Characterization of a β-adrenergic
receptor in porcine trachealis muscle. _Am J Physiol_ 247:C339–C342. Article Google Scholar * Shaul PW, Muntz KH, Buja LM 1990 Comparison ofβ-adrenergic receptor binding characteristics
and coupling to adenylate cyclase in rat pulmonary artery _versus_ aorta. _J Pharmacol Exp Ther_ 252: 86–92. CAS PubMed Google Scholar * Bradford MM 1973 A rapid method for the
quantitation of microgram quantities of protein utilizing the principle of protein dye binding. _Anal Chem_ 72: 249–254. Google Scholar * Lincoln TM, Fisher-Simpson V 1984 A comparison of
the effects of forskolin and sodium nitroprusside on cyclic nucleotides and relaxation in the rat aorta. _Eur J Pharmacol_ 101: 17–27. Article CAS PubMed Google Scholar * Thompson WJ
1991 Cyclic nucleotide phosphodiesterases: pharmacology, biochemistry and function. _Pharmacol Ther_ 51: 13–33. Article CAS PubMed Google Scholar * Meyer RBJ, Miller JP 1974 Analogs of
cyclic AMP and cyclic GMP: general methods of synthesis and the relationship of structure to enzymic activity. _Life Sci_ 14: 1019–1040. Article CAS PubMed Google Scholar * Martinez AM,
Padbury JF, Humme JA, Evan CW, Shames L 1990 Plasma catecholamine and their physiological thresholds during the first ten days of life in sheep. _J Dev Physiol_ 13: 141–146. CAS PubMed
Google Scholar * Padbury JF, Ludlow JK, Ervin MG, Jacobs HC, Humme JA 1987 Threshold for physiological effects of plasma catecholamine in fetal sheep. _Am J Physiol_ 252:E530–E537. CAS
PubMed Google Scholar * Leffler CW, Hessler JR, Terragno NA 1980 Ventilation-induced release of prostaglandin-like material from fetal lungs. _Am J Physiol_ 238:H282–H286. CAS PubMed
Google Scholar * Badesch DB, Orton EC, Zapp LM, Westcott JA, Hester J, Voelkel NF, Stenmark KR 1989 Decreased arterial wall prostaglandin production in neonatal calves with severe chronic
pulmonary hypertension. _Am J Respir Cell Biol_ 1: 489–498. Article CAS Google Scholar * Coleman RA, Smith WL, Narumiya S 1994 VIII. International Union of Pharmacology classification of
prostanoid receptor: properties, distribution, and structure of the receptors and their subtypes. _Pharmacol Rev_ 46: 205–229. CAS PubMed Google Scholar Download references
ACKNOWLEDGEMENTS The authors gratefully acknowledge the contribution of Drs. M. Gore Ervin, Machiko Ikegami, Alan H. Jobe, James F. Padbury, and Daniel H. Polk to the study. We thank Jean
Morris and James Humme for technical assistance and Nancy Feldman of secretarial help. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pediatrics, Harbor-UCLA Medical Center,
University of California, Los Angeles, School of Medicine, Torrance, 90509, California Yuansheng Gao, Haiyan Zhou, Jean-Francois Tolsa, Hai Shen & J Usha Raj Authors * Yuansheng Gao View
author publications You can also search for this author inPubMed Google Scholar * Haiyan Zhou View author publications You can also search for this author inPubMed Google Scholar *
Jean-Francois Tolsa View author publications You can also search for this author inPubMed Google Scholar * Hai Shen View author publications You can also search for this author inPubMed
Google Scholar * J Usha Raj View author publications You can also search for this author inPubMed Google Scholar ADDITIONAL INFORMATION Supported by the National Institute of Child Health
and Human Development, Grant HD-29713, and le Fonds de Perfectionnement du CHUV, la Societe Academique Vaudoise, Lausanne, Switzerland. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Gao, Y., Zhou, H., Tolsa, JF. _et al._ Antenatal Betamethasone Therapy Augments Isoproterenol and Prostaglandin E2-Mediated Relaxation of Preterm Ovine
Pulmonary Veins. _Pediatr Res_ 42, 545–549 (1997). https://doi.org/10.1203/00006450-199710000-00021 Download citation * Received: 12 August 1996 * Accepted: 16 June 1997 * Issue Date: 01
October 1997 * DOI: https://doi.org/10.1203/00006450-199710000-00021 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry,
a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative








