- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT A new class of aryl trifluorovinyl ether monomers were designed and synthesized, and novel fluorinated polymers containing perfluorocyclobutane and 1_H_-1,2,3-triazole units were
prepared from these monomers. These polymers were characterized by nuclear magnetic resonance spectroscopy, thermo-gravimetric analysis and differential scanning calorimetry. The
temperatures of 5% weight loss of the polymers under nitrogen were up to ∼290 °C, and the glass transition temperatures of the polymers were in the range of 79–110 °C. These new polymers
showed good solubility in common organic solvents such as dimethyl sulfoxide and _N_,_N_-dimethylacetamide. In addition, the proton conductivity of the polymers was measured under anhydrous
conditions using impedance spectroscopy, and a maximum conductivity of 2.85 μS cm−1 was obtained at 200 °C. SIMILAR CONTENT BEING VIEWED BY OTHERS POSTMODIFICATION OF HIGHLY DELOCALIZED
CATIONS IN AN AZIDE-BASED POLYMER VIA COPPER-CATALYZED CYCLOADDITION FOR ANION EXCHANGE MEMBRANES Article 16 November 2022 SYNTHESIS OF PHOSPHINE SULFIDE GROUP-CONTAINING AROMATIC
POLY(ETHER)S WITH ALIPHATIC SUBSTITUENTS ON THE PHOSPHORUS ATOMS AND LOW DIELECTRIC PROPERTIES Article 08 August 2024 B-N LEWIS PAIR-FUSED DIPYRIDYLFLUORENE COPOLYMERS INCORPORATING
ELECTRON-DEFICIENT BENZOTHIADIAZOLE COMONOMERS Article 16 November 2022 INTRODUCTION Proton-conducting polymers have attracted much attention as promising materials for membranes for fuel
cells. The operation of proton exchange membrane fuel cells at medium temperatures (100–200 °C) can provide many advantages, such as improved carbon monoxide tolerance, higher energy
efficiency and simplified heat management.1, 2, 3, 4 Thus far, many efforts have been made to develop proton-conducting materials for medium temperature fuel cells.5, 6, 7, 8 One of these
approaches involves finding compounds with an amphoteric nature to act as water substitutes. Recently, nitrogen-containing heterocyclic compounds, such as imidazole, pyrazole and
benzimidazole, have been suggested as proton solvents for proton exchange membrane fuel cells, and they may also be covalently tethered to some suitable polymers to prepare fully polymeric
materials.9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Liu _et al._19 suggested that 1_H_-1,2,3-triazole has adequate electrochemical stability for fuel cell applications, and the
1_H_-1,2,3-triazole groups immobilized on a polymer backbone may open up new avenues in the design of polyelectrolytes. To date, several polymeric systems with tethered 1_H_-1,2,3-triazole
moieties have been evaluated as polyelectrolytes.13, 14, 15, 16, 17 However, most of these polymers derived from polyacrylate or polyethylene may have issues related to their electrochemical
or dimensional stability for medium temperature fuel cells. During the past decade, partially fluorinated polymers containing perfluorocyclobutane (PFCB) rings have been developed.19, 20,
21 These polymers exhibit high-thermal/oxidative/mechanical stability, good chemical resistance and flexibility. In addition, it was recently reported that this type of polymer could be
promising for use in the membranes of fuel cells.22, 23 From this point of view, we have been trying to develop new partially fluorinated ionomers into which both PFCB and
1_H_-1,2,3-triazole units are incorporated. In these ionomers, triazole units are covalently grafted to the polymer backbone containing PFCB groups by flexible chains, which may increase the
mobility of triazole units to facilitate proton transfer. In this work, we report the synthesis and characterization of novel polymers containing PFCB and 1_H_-1,2,3-triazoles moieties. The
polymer backbones and 1_H_-1,2,3-triazoles units are connected by flexible oligo(ethylene oxide) chains. These polymers were prepared from novel aromatic trifluorovinyl ether monomers (6)
via thermal (2π+2π) cyclopolymerization. The preparation process, the corresponding composition and structure, and some physical properties of these polymers were investigated. EXPERIMENTAL
PROCEDURE MATERIALS All chemicals were purchased from Sigma-Aldrich (Sigma-Aldrich Shanghai Trading Co. Ltd., Shanghai, China) with the exception of 1,2-dibromotetrafluoroethane (Shanghai
Sinofluoro Scientific Co., Ltd, Shanghai, China). Acetonitrile and dimethyl sulfoxide (DMSO) were purified by standard methods before use. Zinc turnings were activated by treating with 5%
aqueous hydrochloric acid. Propargyl mono-terminated oligo(ethylene oxide)s (3) and azidomethyl pivalate were prepared using previously published methods.24, 25 Other reagents or materials
were used as received. MEASUREMENTS Gas chromatography/mass spectrometry was recorded on a Finnigan-MAT-8430 (Thermo Finnigan MAT GmbH, Bremen, Germany) instrument using EI ionization at 70
eV. Elemental analysis was carried out on a Carlo-Erba1106 system (Elementar Analysensysteme GmbH, Hanau, Germany). Infrared spectra (IR) were obtained on a Thermo Electron Nicolet Avatar
360 FT-IR spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). 1H nuclear magnetic resonance (NMR; 400 MHz), 13C NMR (100 MHz) and 19F NMR (376 MHz) spectra were recorded on a
Bruker Ultrashield 400 spectrometer (Bruker BioSpin AG, Fällanden, Switzerland). The relative molecular weights and distributions were measured with a Viscotek VE2001 GPC max system (Malvern
Instruments, Worcestershire, UK). The system was calibrated with polystyrene standards. Differential scanning calorimetry (DSC) and thermo-gravimetric analysis (TGA) were conducted on a
PerkinElmer 7 system (PerkinElmer, Waltham, MA, USA). Samples were heated from −50 to 250 °C for DSC and 50–800 °C for TGA at 10 °C min−1 under nitrogen. Proton conductivity of the polymers
was measured using a previously published method.17 SYNTHESIS OF MONOMERS (6) PREPARATION OF 1,3-BIS(2-BROMOTETRAFLUOROETHOXY)ANISOLE (1) A mixture of 5-methoxyresorcinol (14.0 g, 0.100
mol), 1,2-dibromotetrafluoroethane (77.7 g, 0.300 mol) and Cs2CO3 (97.8 g, 0.300 mol) in dry DMSO (250 ml) was stirred for 3 days at 50 °C. After cooling to room temperature, H2O (300 ml)
and CH2Cl2 (100 ml) were added. The aqueous phase was separated and washed with CH2Cl2 (2 × 20 ml). The combined organic phases were washed with H2O (2 × 30 ml), brine (2 × 20 ml), and then
dried over anhydrous MgSO4. After the removal of the solvent, the residue was purified by silica column chromatography using hexane to give compound 1 as a liquid (30.1 g, 61%). 1H NMR
(CDCl3; p.p.m.) _δ_ 6.71 (s, 3H), 3.82 (s, 3H). 13C NMR (CDCl3; p.p.m.) _δ_ 161.1, 149.8, 115.8 (tt, 1C), 113.4 (tt, 1C), 107.5, 106.3, 56.0. 19F NMR (CDCl3; p.p.m.) _δ_ −67.9 (t, 1F), −86.0
(t, 1F). MS: _m_/_z_ 500, 498, 496. Anal. calcd for C11H6Br2F8O3: C, 26.53%; H, 1.21%. Found: C, 26.47%; H, 1.17%. PREPARATION OF 1,3-BIS(2-BROMOTETRAFLUOROETHOXY)PHENOL (2) To a solution
of 1 (10 g, 20 mmol) in anhydrous CH2Cl2 (50 ml), boron tribromide in CH2Cl2 (1 M, 60 ml) was added slowly with stirring at 0 °C. The resulting mixture was allowed to warm to room
temperature and stirred for 48 h. The solution was hydrolyzed by carefully adding H2O (50 ml). The aqueous phase was separated and washed with CH2Cl2 (2 × 10 ml). The combined organic phases
were washed with 5% aqueous NaHCO3 (2 × 10 ml) and H2O (2 × 10 ml) and dried over anhydrous MgSO4. After removal of the solvent, the residue was purified by silica column chromatography
using EtOAc-hexane (1:6, v/v) to give compound 2 as a liquid (9.3 g, 98%). 1H NMR (CDCl3; p.p.m.) _δ_ 6.71 (t, 3H), 5.85 (s, 1H). 13C NMR (CDCl3; p.p.m.) _δ_ 157.1, 149.9, 115.8 (tt, 1C),
113.3 (tt, 1C), 107.7. 19F NMR (CDCl3; p.p.m.) _δ_ −68.3 (t, 1F), −86.4 (t, 1F). IR (neat; cm−1) 3392 (–OH). MS: _m_/_z_ 486, 484, 482. Anal. calcd for C10H4Br2F8O3: C, 24.82%; H, 0.83%.
Found: C, 24.80%; H, 0.82%. PREPARATION OF COMPOUND 4 Compound 3 (10 mmol) and azidomethyl pivalate (1.57 g, 10 mmol) were suspended in tetrahydrofuran (THF)-H2O (20 ml, 1:1, v/v). To this
mixture copper (II) sulfate pentahydrate (0.125 g, 0.5 mmol) and sodium ascorbate (0.594 g, 3 mmol) were added. The mixture was stirred at room temperature for 48 h. The resulting mixture
was diluted with H2O (20 ml) and then extracted with EtOAc (2 × 20 ml). The combined organic layers were washed with water (2 × 10 ml) and brine (2 × 10 ml) and dried over anhydrous MgSO4.
After the removal of solvent, the residue was purified by silica column chromatography using hexane-EtOAc (1:3, v/v) to give compound 4 as a colorless oil. Compound 4A: Yield 96%. 1H NMR
(CDCl3; p.p.m.) _δ_ 7.80 (s, 1H), 7.77 (d, 2H), 7.30 (d, 2H), 6.19 (s, 2H), 4.62 (s, 2H), 4.11 (t, 2H), 3.53–3.65 (m, 6H), 2.39 (s, 3H), 1.14 (s, 9H). IR (neat; cm−1) 1745 (C=O). MS: _m_/_z_
455. Compound 4B: Yield 93%. 1H NMR (CDCl3; p.p.m.) _δ_ 7.67 (d, 1H), 7.54 (d, 2H), 7.13 (d, 2H), 6.01 (s, 2H), 4.43 (s, 2H), 3.91 (t, 2H), 3.33–3.45 (m, 10H), 2.19 (s, 3H), 0.93 (s, 9H).
IR (neat; cm−1) 1744 (C=O). MS: _m_/_z_ 499. Compound 4C: Yield 92%. 1H NMR (CDCl3; p.p.m.) _δ_ 7.78 (d, 1H), 7.74 (d, 2H), 7.29 (d, 2H), 6.16 (s, 2H), 4.63 (s, 2H), 4.09 (t, 2H), 3.55–3.70
(m, 14H), 2.38 (s, 3H), 1.12 (s, 9H). IR (neat; cm−1) 1744 (C=O). MS: _m_/_z_ 543. PREPARATION OF COMPOUND 5 A mixture of 2 (4.84 g, 0.01 mol), 4 (0.01 mol) and anhydrous K2CO3 (2.76 g, 0.02
mol) in CH3CN (50 ml) was stirred at reflux for 48 h. After the removal of the solvent, the residue was diluted with H2O and EtOAc. The organic layer was separated, and the aqueous layer
was extracted with EtOAc (2 × 10 ml). The combined organic layers were washed with H2O (2 × 10 ml) and brine (2 × 10 ml) and dried over anhydrous MgSO4. After the removal of the solvent, the
residue was purified by silica column chromatography using hexane-EtOAc (2:7, v/v) to give compound 5 as a colorless oil. Compound 5A: Yield 89%. 1H NMR (CDCl3; p.p.m.) _δ_ 7.79 (s, 1H),
6.73 (d, 3H), 6.19 (s, 2H), 4.68 (s, 2H), 4.13 (t, 2H), 3.84 (t, 2H), 3.71 (s, 4H), 1.16 (s, 9H). 19F NMR (CDCl3; p.p.m.) _δ_ −67.9 (t, 1F), −86.0 (t, 1F). MS: _m_/_z_ 769, 767, 765. Anal.
calcd for C23H25Br2F8N3O7: C, 36.00%; H, 3.28%. Found: C, 35.95%; H, 3.25%. Compound 5B: Yield 87%. 1H NMR (CDCl3; p.p.m.) _δ_ 7.67 (s, 1H), 6.72 (d, 3H), 6.20 (s, 2H), 4.63 (s, 2H), 4.11
(t, 2H), 3.82 (t, 2H), 3.64–3.68 (m, 8H), 1.14 (s, 9H). 19F NMR (CDCl3; p.p.m.) _δ_ −67.9 (t, 1F), −86.0 (t, 1F). _δ_ 21.3. MS: _m_/_z_ 813, 811, 809. Anal. calcd for C25H29Br2F8N3O8: C,
37.01%; H, 3.60%. Found: C, 36.97%; H, 3.57%. Compound 5C: Yield 83%. 1H NMR (CDCl3; p.p.m.) _δ_ 7.69 (s, 1H), 6.73 (d, 3H), 6.21 (s, 2H), 4.64 (s, 2H), 4.12 (t, 2H), 3.83 (t, 2H), 3.52–3.73
(m, 12H), 1.16 (s, 9H). 19F NMR (CDCl3; p.p.m.) _δ_ −67.9 (t, 1F), −86.0 (t, 1F). MS: _m_/_z_ 857, 855, 853. Anal. calcd for C27H33Br2F8N3O9: C, 37.91%; H, 3.89%. Found: C, 37.88%; H,
3.87%. PREPARATION OF COMPOUND 6 To a flame-dried 100-ml flask was added 5 (10 mmol), zinc (1.95 g, 30 mmol) and CH3CN (30 ml). The resulting mixture was stirred at reflux for 48 h. After
removal of the solvent, the residue was diluted with CH2Cl2 and then filtered. The filtrate was washed with H2O and brine and dried over anhydrous MgSO4. After removal of the solvent, the
residue was purified by silica column chromatography using hexane-EtOAc (1:3, v/v) to give compound 6 as a colorless oil. Compound 6A: Yield 77%. MS: _m_/_z_ 569. Anal. calcd for
C23H25F6N3O7: C, 48.51%; H, 4.43%. Found: C, 48.50%; H, 4.43%. Compound 6B: Yield 75%. MS: _m_/_z_ 613. Anal. calcd for C25H29F6N3O8: C, 48.94%; H, 4.76%. Found: C, 48.92%; H, 4.75%.
Compound 6C: Yield 72%. MS: _m_/_z_ 657. Anal. calcd for C27H33F6N3O9: C, 49.32%; H, 5.06%. Found: C, 49.30%; H, 5.05%. SYNTHESIS OF POLYMERS 7 AND 8 Compound 6 (1 mmol) was added to a
pre-dried 50-ml flask. After three cycles of freeze-pump-thaw, the flask was heated at 190 °C for 48 h. After cooling to room temperature, the residue was dissolved in CHCl3 (10 ml) and then
precipitated in hexane to give polymer 7 (90–95%). To a solution of polymer 7 (0.5 g) in methanol (10 ml) aqueous NaOH (1 M, 1 ml) was added. The resulting mixture was stirred at room
temperature for 12 h and subsequently neutralized with 5% aqueous hydrochloric acid. The solution was concentrated under vacuum and then dissolved in methanol (5 ml). The resulting solution
was triturated with excess diethyl ether. After filtration, the obtained solid was dried at 100 oC for 24 h to give polymer 8 (82–87%). RESULTS AND DISCUSSION MONOMER SYNTHESES The
preparation procedure for the monomers (6) is shown in Scheme 1. The synthesis of 1 initially focused on traditional methods.23, 24, 25 However, 6 was obtained in very low yield (<20%).
Most of the products were derived from the bromination of the benzene ring, which was confirmed by 1H NMR and gas chromatography/mass spectrometry. Following a new route,26 the yield of 6
could be increased to 61%. In this route, the phenoxides were generated _in situ_, which not only avoided using harsh conditions to prepare moisture sensitive phenoxides but also gave the
desired products in higher yield.26 Using boron tribromide, demethylation of 1 was achieved successfully with relatively high yield (>96%). Treatment of 3 with azidomethyl pivalate gave 4
in high yield (92–96%) by a typical ‘click’ reaction. Using potassium carbonate as a base, the coupling reaction between 4 and 2 proceeded smoothly to give 5 in good yield (83–89%). In the
last step, 6 was synthesized from 5 by zinc-mediated elimination in anhydrous acetonitrile. The composition and structure of 6 were confirmed by Fourier transform infrared (FT-IR), 1H-, 13C-
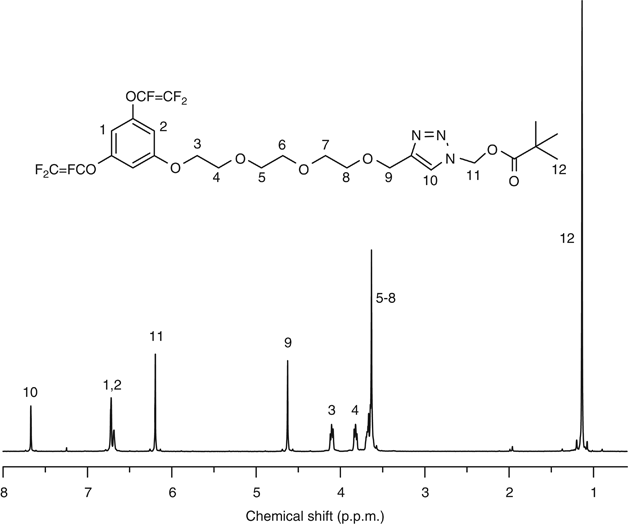
and 19F-NMR. Compound 6B is discussed as a typical example. In the 1H NMR spectrum of 6B (Figure 1), the signals of methyl (at 1.13 p.p.m.) and ethylene (at 6.21 p.p.m.) in the
pivaloyloxymethyl (POM) moieties were observed, and the singlet peak at 4.63 p.p.m. indicated the two protons adjacent to the triazole ring. In the 13C NMR spectrum of 6B (Figure 2), the
typical signals of –OCF=CF2 groups were detected. The signals between 134.0 and 135.5 p.p.m. and 143.0 and 151.5 p.p.m. were attributed to the units of –O_C_F= and =_C_F2, respectively.
These peaks overlapped with the signals of the carbon atoms in triazole rings, which appeared at 147.2 and 135.5 p.p.m. In addition, the four peaks at 26.9, 38.9, 74.2 and 177.0 p.p.m. were
assigned to the carbon atoms in the POM moiety. The 19F NMR spectrum (Figure 3a) exhibited three doublets centered at −119.0, −125.7 and −134.6 p.p.m., which were assigned to the fluorine
atoms in –OCF=CF2. Figure 5a displays the FT-IR spectrum of 6B. The sharp band at 1832 cm−1 is the typical signal of –OCF=CF2 groups, and the strong band at 1746 cm−1 was attributed to the
carbonyl in the POM moieties. The characterization of 6 by gas chromatography/mass spectrometry and elemental analysis further confirmed the successful synthesis of the high-purity monomers.
POLYMER SYNTHESES The preparation procedures of polymers 7 and 8 are shown in Scheme 1. polymer 7 was prepared by the thermal (2π+2π) cyclopolymerization of 6 in bulk. This polymerization
process did not require catalysts or initiators and gave polymer 7 in high yield. The formation of polymer 7 was confirmed by FT-IR, 1H- and 19F NMR. Polymer 7B is discussed as a typical
example. In the FT-IR spectrum of polymer 7B (Figure 4b), the sharp band at 1834 cm−1 disappeared, and a new band at 989 cm−1 appeared, which represented the PFCB groups. In Figure 3b, the
resonance signals of the –OCF=CF2 groups disappeared, and a series of new peaks between −126.7 and −132.1 p.p.m. appeared, which are typical fluorine signals of PFCB groups. The molecular
weights of polymer 7 determined by gel permeation chromatography in THF using polystyrene as the standard ranged from 37 900 to 38 800 for _M_w with _M_w/_M_n values of 2.5–2.8 (Table 1).
Treatment of polymer 7 with NaOH gave polymer 8 in good yield. The conversion of polymer 7 to polymer 8 was confirmed by 1H NMR and FT-IR spectra. The disappearance of the peaks at 1.14 and
6.20 p.p.m. (Figure 5b) indicated the successful removal of the POM moieties, which was further confirmed by the FT-IR spectrum of polymer 8B (Figure 4c). In Figure 4c, a broad band from
2500 to 3500 cm−1 was attributed to the –NH groups in 1_H_-1,2,3-triazole moieties, and the disappearance of a strong band at 1746 cm−1 indicated the absence of the carbonyl groups in the
POM moieties. PROPERTIES OF POLYMERS SOLUBILITY OF POLYMERS Table 1 shows solubility of the polymers determined quantitatively by dissolving 0.1 g polymer in 1.0 g organic solvents. Polymer
7 showed good solubility in common organic solvents such as DMSO, CHCl3 and THF. After removal of POM moieties, however, the resulting polymers (polymer 8) were not soluble in solvents with
low dielectric constants and non-polar solvents such as THF and CHCl3, which might be attributed to the strong hydrogen-bonding action between the 1_H_-1,2,3-triazole and oligo(ethylene
oxide) units. THERMAL PROPERTIES OF POLYMERS The thermal properties of the polymers were evaluated by DSC and TGA. As shown in Table 1, the glass transition temperature (_T_g) values of
polymer 8 were much higher than those of polymer 7, and the _T_g value (polymer 8) distinctly decreased with the reduction of the triazole content, which was attributed to the highly
hydrogen bonded network formed between the triazole and oligo(ethylene oxide) units. The weight percent of triazole contained in each polymer was calculated by dividing the equivalent weight
of the triazole units (68 g mol−1) by the equivalent weight of the polymer repeat unit. Figure 6 shows the TGA traces of polymer 8 under nitrogen. These polymers were stable up to ∼290 °C,
which is much higher than that of previously reported polymers.16, 17 PROTON CONDUCTIVITY The proton conductivity of polymer 8 was measured from 40 to 200 °C under anhydrous conditions using
impedance spectroscopy. In general, the proton conductivity of polymers containing _N_-heterocyclic moieties is known to be influenced by several factors such as the glass transition
temperature of the polymer and the proton charge carrier density.12, 13, 14 As expected, polymer 8 exhibited very similar conductivity (Figure 7) because they possessed identical polymeric
structure. A maximum conductivity of 2.85 μS cm−1 was obtained at 200 °C under anhydrous conditions. The proton conductivities of polymer 8 were lower than the values previously reported,16,
17 which could be attributed to the higher _T_g and lower triazole content of polymer 8. The introduction of more than one triazole per repeat unit did not result in an increase in
conductivity because there was an accompanying increase in _T_g;16 thus, two methods are suggested to improve the proton conductivity. The first one is the reduction of _T_g by the
incorporation of poly(ethylene oxide) (PEO) blocks to prepare copolymers. The second one is the increase of the acidity via the incorporation of electron withdrawing groups such as a
trifluoromethyl group into the triazole rings. CONCLUSIONS A new class of perfluorocyclobutyl poly(arylene ether)s containing oligo(ethylene oxide) and 1_H_-1,2,3-triazole units were
prepared and characterized. TGA show that these polymers had good thermal stability. DSC analysis indicated that the _T_g value of these polymers decreased with the triazole content. These
polymers also showed good solubility in common organic solvents such as DMSO and DMF. All polymers exhibited a proton conductivity comparable to that previously reported.16 Systematic
investigations of these polymers as solid electrolyte membranes for fuel cells applications are in progress. REFERENCES * Zhang, J., Xie, Z., Zhang, J., Tang, Y., Song, C., Navessin, T.,
Shi, Z., Song, D., Wang, H., Wilkinson, D. P., Liu, Z.- S. & Holdcroft, S. High temperature PEM fuel cells. _J. Power Sources_ 160, 872–891 (2006). Article CAS Google Scholar * de
Bruijn, F. A., Makkus, R. C., Mallant, R.K.A.M. & Janssen, G. J. M. Chapter five materials for state-of-the-art PEM fuel cells, and their suitability for operation above 100 °C. _Adv.
Fuel Cells_ 1, 235–336 (2007). Article CAS Google Scholar * Mader, J., Xiao, L., Schmidt, T. J. & Benicewicz, B. C. Polybenzimidazole/acid complexes as high-temperature membranes.
_Adv. Polym. Sci._ 216, 63–124 (2008). CAS Google Scholar * Li, Q., He, R., Jensen, J. O. & Bjerrum, N. J. Approaches and recent development of polymer electrolyte membranes for fuel
cells operating above 100 °C. _Chem. Mater._ 15, 4896–4915 (2003). Article CAS Google Scholar * Wang, J. T., Savinell, R. F., Wainright, J., Litt, M. & Yu, H. A H2/O2 fuel cell using
acid doped polybenzimidazole as polymer electrolyte. _Electrochim. Acta._ 41, 193–197 (1996). Article CAS Google Scholar * Bozkurt, A., Ise, M., Kreuer, K. D., Meyer, W. H. & Wegner,
G. Proton conducting polymer electrolytes based on phosphoric acid. _Solid State Ionics_ 125, 225–233 (1999). Article CAS Google Scholar * Miyake, N., Wainright, J. S. & Savinell, R.
F. Evaluation of a sol-gel derived Nafion/silica hybrid membrane for proton electrolyte membrane fuel cell applications. _J. Electrochem. Soc._ 148, A898–A904 (2001). Article CAS Google
Scholar * Pu, H., Liu, Q. & Liu, G. Methanol permeation and proton conductivity of acid-doped poly(_N_-ethylbenzimidazole) and poly(_N_-methylbenzimidazole). _J. Membr. Sci._ 241,
169–175 (2004). Article CAS Google Scholar * Schuster, M., Meyer, W. H., Wegner, G., Herz, H. G., Ise, M., Schuster, M., Kreuer, K. D. & Maier, J. Proton mobility in oligomer-bound
proton solvents: imidazole immobilization via flexible spacers. _Solid State Ionics_ 145, 85–92 (2001). Article CAS Google Scholar * Persson, J. C. & Jannasch, P. Self-conducting
benzimidazole oligomers for proton transport. _Chem. Mater._ 15, 3044–3045 (2003). Article CAS Google Scholar * Jeske, M., Soltmann, C., Ellenberg, C., Wilhelm, M., Koch, D. &
Grathwoh, G. Proton conducting membranes for the high temperature polymer electrolyte membrane fuel cell (HT-PEMFC) based on functionalized polysiloxanes. _Fuel Cells_ 7, 40–46 (2007).
Article CAS Google Scholar * Woudenberg, R. C., Yavuzcetin, O., Tuominen, M. T. & Coughlin, E. B. Intrinsically proton conducting polymers and copolymers containing benzimidazole
moieties: glass transition effects. _Solid State Ionics_ 178, 1135–1141 (2007). Article CAS Google Scholar * Zhou, Z., Li, S., Zhang, Y., Liu, M. & Li, W. Promotion of proton
conduction in polymer electrolyte membranes by 1_H_-1,2,3-triazole. _J. Am. Chem. Soc._ 127, 10824–10825 (2005). Article CAS Google Scholar * Subbaraman, R., Ghassemi, H. &
Zawodzinski, T. A. Jr 4,5-Dicyano-1_H_-[1,2,3]-triazole as a proton transport facilitator for polymer electrolyte membrane fuel cells. _J. Am. Chem. Soc._ 129, 2238–2239 (2007). Article CAS
Google Scholar * Martwiset, S., Woudenberg, R. C., Granados-Focil, S., Yavuzcetin, O., Tuominen, M. T. & Coughlin, E. B. Intrinsically conducting polymers and copolymers containing
triazole moieties. _Solid State Ionics_ 178, 1398–1403 (2007). Article CAS Google Scholar * Martwiset, S., Yavuzcetin, O., Thorn, M., Versek, C., Tuominen, M. & Coughlin, E. B. Proton
conducting polymers containing 1_H_-1,2,3-triazole moieties. _J. Polym. Sci. Part A Polym. Chem._ 47, 188–196 (2009). Article CAS Google Scholar * Nagamani, C., Versek, C., Thorn, M.,
Tuominen, M. T. & Thayumanavan, S. Proton conduction in 1_H_-1,2,3-triazole polymers: imidazole-like or pyrazole-like? _J. Polym. Sci. Part A Polym. Chem._ 48, 1851–1858 (2010). Article
CAS Google Scholar * Frutsaert, G., Delon, L., David, D., Ameduri, B., Jones, D., Glipa, X. & Roziers, J. Synthesis and properties of new fluorinated polymers bearing pendant
imidazole groups for fuel cell membranes operating over a broad relative humidity range. _J. Polym. Sci. Part A Polym. Chem._ 48, 223–231 (2010). Article CAS Google Scholar * Babb, D. A.,
Ezzell, B. R., Clement, K. S., Richey, W. F. & Kennedy, A. P. Perfluorocyclobutane aromatic ether polymers. _J. Polym. Sci. Part A Polym. Chem._ 31, 3465–3477 (1993). Article CAS
Google Scholar * Smith, D. W. Jr, Babb, D. A., Shah, H. V., Hoeglund, A., Traiphol, R., Perahia, D., Boone, H. W., Langhoff, C. & Radler, M. Perfluorocyclobutane (PFCB) polyaryl ethers:
versatile coatings materials. _J. Fluorine Chem._ 104, 109–117 (2000). Article CAS Google Scholar * Zhu, Y., Huang, Y., Meng, W.- D., Li, H. & Qing, F.- L. Novel perfluorocyclobutyl
(PFCB)-containing polymers formed by click chemistry. _Polymer_ 47, 6272–6279 (2006). Article CAS Google Scholar * Ford, L. A., DesMarteau, D. D. & Smith, D. W. Jr Perfluorocyclobutyl
(PFCB) aromatic polyethers: synthesis and characterization of new sulfonimide containing monomers and fluoropolymers. _J. Fluorine Chem._ 126, 651–658 (2005). Article Google Scholar *
Kim, D.- J., Chang, B.- J., Kim, J.- H., Lee, S.- B. & Joo, H.- J. Sulfonated poly(fluorenyl ether) membranes containing perfluorocyclobutane groups for fuel cell applications. _J.
Membrane Sci._ 325, 217–222 (2008). Article CAS Google Scholar * Lu, G., Lam, S. & Burgess, K. An iterative route to ‘decorated’ ethylene glycol-based linkers. _Chem. Commun._ 2006,
1652–1654 (2006). Article Google Scholar * Loren, J. C., Krasinski, A., Fokin, V. V. & Sharpless, K. B. _NH_-1,2,3-triazoles from azidomethyl pivalate and carbamates: base-labile
_N_-protecting groups. _Synlett_ 2005, 2847–2850 (2005). Google Scholar * Li, J., Qiao, J., Smith, D., Chen, B., Salvati, M. E., Roberge, J. Y. & Balasubramanian, B. N. A practical
synthesis of aryl tetrafluoroethyl ethers via the improved reaction of phenols with 1,2-dibromotetrafluoroethane. _Tetrahedron Lett._ 48, 7516–7519 (2007). Article CAS Google Scholar
Download references ACKNOWLEDGEMENTS We are grateful to the Doctoral Fund of the Ministry of Education of China (200802551014) and the Open Foundation of State Key Laboratory for
Modification of Chemical Fibers and Polymer Materials (LK0804) for financial support. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Chemistry, College of Chemistry, Chemical
Engineering and Biotechnology, Donghua University, Shanghai, PR China Yuanqin Zhu & Chuanglong He * Department of Applied Physics, College of Physical Science and Technology, Guangxi
University, Guangxi, PR China Haihua Chen Authors * Yuanqin Zhu View author publications You can also search for this author inPubMed Google Scholar * Haihua Chen View author publications
You can also search for this author inPubMed Google Scholar * Chuanglong He View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHORS
Correspondence to Yuanqin Zhu or Chuanglong He. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhu, Y., Chen, H. & He, C. Novel polyelectrolytes
containing perfluorocyclobutane and triazole units: synthesis, characterization and properties. _Polym J_ 43, 258–264 (2011). https://doi.org/10.1038/pj.2010.129 Download citation *
Received: 17 August 2010 * Revised: 03 November 2010 * Accepted: 08 November 2010 * Published: 22 December 2010 * Issue Date: March 2011 * DOI: https://doi.org/10.1038/pj.2010.129 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * fluorinated polymers * oligo(ethylene oxide) * polyelectrolytes * triazole








