- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The crizotinib-resistant _ALK__F1174L_ mutation arises _de novo_ in neuroblastoma (NB) and is acquired in _ALK_ translocation-driven cancers, lending impetus to the development of
novel anaplastic lymphoma kinase (ALK) inhibitors with different modes of action. The diaminopyrimidine TAE684 and its derivative ceritinib (LDK378), which are structurally distinct from
crizotinib, are active against NB cells expressing _ALK__F1174L_. Here we demonstrate acquired resistance to TAE684 and LDK378 in ALKF1174L-driven human NB cells that is linked to
overexpression and activation of the AXL tyrosine kinase and epithelial-to-mesenchymal transition (EMT). AXL phosphorylation conferred TAE684 resistance to NB cells through upregulated
extracellular signal-regulated kinase (ERK) signaling. Inhibition of AXL partly rescued TAE684 resistance, resensitizing these cells to this compound. AXL activation in resistant cells was
mediated through increased expression of the active form of its ligand, GAS6, that also served to stabilize the AXL protein. Although ectopic expression of _AXL_ and _TWIST2_ individually in
TAE684-sensitive parental cells led to the elevated expression of mesenchymal markers and invasive capacity, only _AXL_ overexpression induced resistance to TAE684 as well. TAE684-resistant
cells showed greater sensitivity to HSP90 inhibition than did their parental counterparts, with downregulation of AXL and AXL-mediated ERK signaling. Our studies indicate that aberrant AXL
signaling and development of an EMT phenotype underlie resistance of ALKF1174L-driven NB cells to TAE684 and its derivatives. We suggest that the combination of ALK and AXL or HSP90
inhibitors be considered to delay the emergence of such resistance. SIMILAR CONTENT BEING VIEWED BY OTHERS GILTERITINIB OVERCOMES LORLATINIB RESISTANCE IN _ALK_-REARRANGED CANCER Article
Open access 24 February 2021 ITGB1 AND DDR ACTIVATION AS NOVEL MEDIATORS IN ACQUIRED RESISTANCE TO OSIMERTINIB AND MEK INHIBITORS IN EGFR-MUTANT NSCLC Article Open access 04 January 2024
INCREASED MCL1 DEPENDENCY LEADS TO NEW APPLICATIONS OF BH3-MIMETICS IN DRUG-RESISTANT NEUROBLASTOMA Article Open access 19 September 2023 INTRODUCTION The predictable emergence of resistance
to tyrosine kinase inhibitors (TKIs), leading to disease progression or relapse, has hindered their long-term therapeutic impact.1 This obstacle is best exemplified by the development of
resistance to imatinib in _BCR-ABL_-expressing chronic myeloid leukemia and gefitinib in _EGFR_-mutant non-small-cell lung cancer,2, 3 and is likely to impede efforts to devise effective
targeted therapy for many other cancers, including neuroblastoma (NB). This aggressive childhood tumor is characterized by mutations in the anaplastic lymphoma kinase (ALK) receptor tyrosine
kinase (RTK) in 10% of cases.4, 5, 6, 7 Many of these point mutations are considered ‘drivers’ of the malignant process: not only do they induce constitutive, ligand-independent activation
of ALK signaling, but also their inhibition leads to tumor cell death and tumor regression.5, 6 The most common somatic mutation in NB, _ALK__F1174L_, is highly tumorigenic, both by itself
and when coexpressed with the _MYCN_ oncogene, a combination that increases the penetrance of the disease and accelerates tumor formation.8, 9 This mutation confers primary resistance to the
ALK inhibitor crizotinib in NB9 and serves as a mechanism of acquired resistance to crizotinib in patients with _ALK_-translocated cancers.10 Several structurally unrelated small molecule
ALK inhibitors have been developed such as alectinib (CH5424802) that has shown activity against _ALK__F1174L_-positive tumors.11 Similarly, the lead compound TAE684,12 from which the
recently Food and Drug Administration (FDA)-approved inhibitor ceritinib (LDK378, Novartis) is derived,13 has exhibited potent activity against ALKF1174L in both _ALK_ translocation-positive
cancers10 and NB.5, 14 Nonetheless, resistance to these ATP-competitive agents will inevitably develop as a consequence of their wider clinical application. We therefore sought to elucidate
the mechanism(s) underlying acquired resistance to ALK inhibitors in ALKF1174L-driven NB as a means to uncover secondary targets that could be exploited to prolong responses in these
patients. By generating TAE684 and LDK378 resistance models of _ALK__F1174L_-positive human NB cells, we identified overexpression and GAS6-mediated activation of a TAM family RTK, AXL, as
the principal resistance-related alteration in these cells. This change was associated with activation of the mitogen-activated protein kinase (MAPK) signaling pathway and the development of
an epithelial-to-mesenchymal transition (EMT) phenotype. Importantly, inhibition of AXL with a small molecule inhibitor led to decreased growth and invasiveness of the resistant cells with
a concomitant decrease in extracellular signal-regulated kinase (ERK) signaling. We also demonstrate that HSP90 inhibition, through its impact on AXL binding, induces striking cytotoxicity
in TAE684-resistant cells. Hence, we suggest that the combination of ALK and AXL or HSP90 inhibition could serve as part of an effective strategy of targeted therapeutics for
ALKF1174L-driven NB and other tumors dependent on this aberrant RTK. RESULTS TAE684 RESISTANCE IS ASSOCIATED WITH THE LOSS OF ALK ACTIVITY BUT MAINTENANCE OF DOWNSTREAM SIGNALING NB cells
that express the _ALK__F1174L_ mutation are relatively resistant to crizotinib but are sensitive to TAE684.5, 9 To elucidate the mechanisms of resistance to ALKF1174L inhibitors, we first
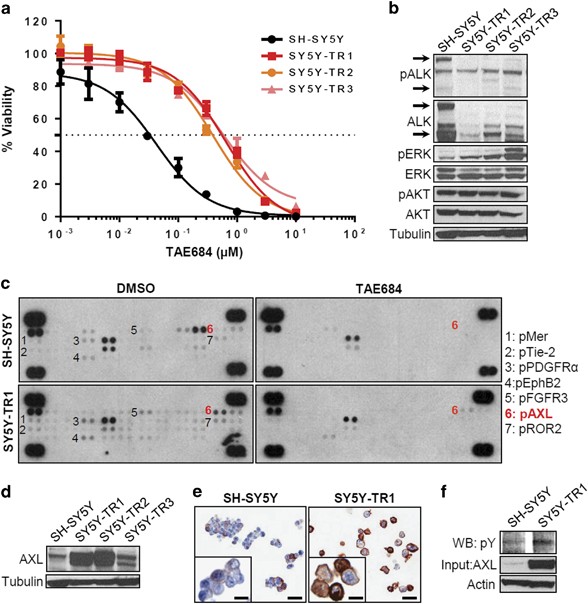
established TAE684-resistant cells (SH-SY5Y-TR) through continuous exposure of SH-SY5Y cells to increasing doses of the compound over 8–12 months (Supplementary Figure S1a). Three individual
subclones (SH-SY5Y-TR1, SH-SY5Y-TR2 and SH-SY5Y-TR3) were expanded (Figure 1a), and subsequently maintained in ∼35 times the half-maximal inhibitory concentration (IC50) of TAE684.
Resistance to targeted therapy can arise from either secondary mutations in the drug target itself or upregulation of compensatory pathways.1 We therefore first analyzed the phosphorylation
status of ALK and its downstream targets in SH-SY5Y-TR-resistant cells. As compared with results in parental cells, ALK phosphorylation was decreased in both the primary resistant pool
(Supplementary Figure S1b) and the three subclones (Figure 1b), suggesting that the acquired resistance was not mediated by secondary mutations in _ALK_. This result was confirmed by
sequence analysis and genomic PCR showing the absence of mutations other than _F1174L_ or gene amplification, respectively (data not shown). The absence of ALK phosphorylation also ruled out
upregulation of drug efflux transporters such as the ABC (ATP-binding cassette) superfamily as a potential mechanism of resistance, as ALK would remain phosphorylated if this were to be the
case. The _ALK__F1174L_ mutation activates the phosphatidylinositol-3-kinase/AKT/mammalian target of rapamycin (PI3K/AKT/mTOR) and MAPK/ERK pathways in NB cells, both of which are
downregulated when ALK is inhibited.5, 9 Despite decreased levels of pALK, AKT activation was maintained in the resistant SH-SY5Y-TR pool as well as all three SH-SY5Y-TR subclones (Figure 1b
and Supplementary Figure S1b). Importantly, compared with parental cells, ERK phosphorylation was increased in SH-SY5Y-TR cells and its subclones (Figure 1b and Supplementary Figure S1b).
The upregulated ERK signaling in the context of suppressed ALK phosphorylation suggested the development of an alternative mechanism of resistance, most likely activation of another tyrosine
kinase capable of bypassing TAE684 inhibition. THE AXL RECEPTOR TYROSINE KINASE IS UPREGULATED IN TAE684-RESISTANT NB CELLS To identify upstream RTKs that may contribute to TAE684
resistance, we compared the phosphorylation status of 42 candidates in SH-SY5Y and SH-SY5Y-TR1 cells before and after treatment with TAE684 (Figure 1c). Parental SH-SY5Y cells showed basal
activation of several RTKs, most of which were decreased or lost upon TAE684 treatment (Figure 1c). Under dimethyl sulfoxide (DMSO) treatment conditions, TAE684-resistant SH-SY5Y-TR1 cells
showed enhanced phosphorylation of seven additional RTKs (MER, TIE-2, PDGFRα, EPHB2, FGFR3, AXL and ROR2). Two of these, MER and AXL, belonged to the same TAM receptor tyrosine kinase family
whose aberrantly elevated signaling has been linked to cancer progression, metastasis and resistance to therapy.15 Acute exposure to TAE684 led to complete loss of phosphorylation of all of
these candidates except for AXL and EPHB2. Thus, the sustained upregulation of AXL and EPHB2 in the SH-SY5Y-TR1-resistant cells suggested a role for these RTKs in mediating resistance to
TAE684. We selected AXL for further study because of the known role of this transmembrane receptor in mediating drug resistance, especially to TKIs.16, 17 We confirmed that AXL expression
was markedly increased in two of the three resistant clones and marginally in the third (Figure 1d). Intense membrane staining of AXL was apparent on immunocytochemical staining of
SH-SY5Y-TR1 cells (Figure 1e). Interestingly, parental SH-SY5Y cells also contained a minor subpopulation of _AXL_-expressing cells compared with TAE684-resistant cells (18 of 100 cells vs
94 of 100 resistant cells), suggesting the pre-existence and clonal selection of cells expressing AXL under drug pressure to compensate for the loss of ALK signaling. Finally, the increased
protein expression was accompanied by phosphorylation of AXL in SH-SY5Y-TR1 cells (Figure 1f), suggesting activation of the tyrosine kinase. Further evidence to support AXL activation as a
key mediator of TAE684 resistance came from experiments with an additional ALKF1174L-driven NB cell line, SK-N-SH. SK-N-SH cells rendered resistant to TAE684 (SK-N-SH-TAE-R) showed
upregulation of AXL as well as ERK signaling despite downregulation of phosphorylated ALK (Supplementary Figures S2a and b). Together, these results indicate that TAE684-resistant NB cells
express higher levels of alternative RTKs such as AXL to compensate for the loss of ALK phosphorylation. AXL ACTIVATION CONFERS RESISTANCE TO TAE684 IN ALKF1174L-DRIVEN NB CELLS To clarify
the role of AXL activation in resistance to TAE684, we first abrogated its expression in SH-SY5Y-TR1 cells using small hairpin RNA (shRNA) knockdown, noting a significant decrease in the
growth of AXL-depleted compared with untransfected or control shRNA-expressing cells (Figure 2a). AXL depletion was associated with a decrease in pERK levels at 72 h compared with
untransfected or control shRNA-expressing cells at the same time point (Figure 2b). SH-SY5Y-TR1 cells were three times more sensitive to the AXL inhibitor R428(ref. 18) than were parental
SH-SY5Y cells (Figure 2c). Similar results were seen in the SK-N-SH-TAE-R cells (Supplementary Figure S2c). Importantly, R428 also restored sensitivity to TAE684 in SH-SY5Y-TR1 cells, with a
combination of the two agents having an additive effect (Figure 2d). Similar effects were not seen in TAE684-sensitive parental SH-SY5Y cells, possibly reflecting the dependency of the
resistant cells on the proliferative and migratory effects of activated AXL. Next, we determined whether overexpression of AXL was sufficient to confer resistance to ALK inhibitors in
_ALK_-mutated NB cells. Ectopic expression of _AXL_ in TAE684-sensitive SH-SY5Y cells resulted in a twofold decrease in sensitivity to TAE684 (Figures 2e and f). Overexpression of _AXL_ also
led to an increase in pERK in these cells (Figure 2g). These findings suggest that AXL overexpression contributes to TAE684 resistance in ALKF1174L-driven NB cells and underscores the
potential of AXL inhibition as a means to sensitize these resistant cells. RESISTANCE TO THE ALK INHIBITOR LDK378 IS ALSO ASSOCIATED WITH AXL ACTIVATION To extend findings with TAE684 to the
more clinically relevant ALK inhibitor LDK378 (ceritinib), we first evaluated the sensitivity of both TAE684-resistant SH-SY5Y-TR1 and SK-N-SH-TAE-R cells to LDK378. These experiments
showed cross-resistance between the two compounds (Figure 3a), suggesting that AXL activation might serve as a mechanism of resistance to newer ALK inhibitors. To support this hypothesis
further, we generated LDK378-resistant SH-SY5Y cells (designated LDK-R-5Y), following the same procedure as used with TAE684 (Figure 3b). Similar to the findings in TAE684-resistant cells,
pALK was downregulated in the LDK-R-5Y cells whereas pERK was upregulated (Figure 3c). Moreover, increased expression of AXL at both the mRNA and protein levels, as well as increased
phosphorylation of AXL, were observed (Figures 3c–e). LDK-R-5Y cells were more sensitive to AXL inhibition by R428 than were the parental cells (Figure 3f). Thus, activation of AXL and ERK
signaling appears to underlie acquired resistance of ALKF1174L-driven NB cells to both TAE684 and LDK378. TAE684-RESISTANT SH-SY5Y-TR1 CELLS DISPLAY FUNCTIONAL EMT FEATURES We noted that
SH-SY5Y-TR1 cells exhibited striking morphological differences when compared with parental SH-SY5Y cells (Figure 4a). Whereas the parental cells were smaller in size with epithelioid
morphology, SH-SY5Y-TR1 cells were elongated, spindle shaped and fibroblast-like, with decreased cell-to-cell contact, consistent with EMT. To determine whether these morphological
alterations had an underlying molecular basis, we compared the gene expression profiles of the SH-SY5Y-TR1 cells with those of parental cells (Figure 4b). In addition to upregulation of
_AXL_ itself, the resistant cells showed significant differential expression of genes with major roles in EMT, with overexpression of the key transcriptional inducers _TWIST2_ and _SNAI2_,
and the characteristic mesenchymal markers vimentin (_VIM_) and fibronectin (_FN1_) (Figure 4b). Gene set enrichment analysis (GSEA) of the differentially expressed genes indicated
significant enrichment of three distinct EMT-related gene signatures (Figure 4c).19, 20, 21 Overexpression of _TWIST2_ in the TAE684-resistant cells was confirmed by quantitative reverse
transcriptase–PCR (qRT–PCR; Figure 4d). Although E-cadherin (_CDH1_), a key marker of the epithelial cell state, was not significantly altered in the expression signatures (Figure 4b),
quantitative PCR analysis demonstrated decreased mRNA levels of this gene as well as increased expression of vimentin in the resistant compared with the parental cells, findings that were
also reflected at the protein level (Figure 4e). SH-SY5Y-TR1 cells also displayed significantly increased invasive properties when compared with parental cells on matrigel assays, attesting
to their metastatic potential (Figure 4f). Together, these results suggest that acquired resistance to TAE684 by _ALK_-mutated NB cells is associated with functional EMT. AXL ACTIVATION
LEADS TO EMT FEATURES AND RESISTANCE TO TAE684 THROUGH DIFFERENT MECHANISMS Although AXL activation and EMT induction have both been linked to TKI resistance,15, 22 it is not clear whether
EMT induction is an inevitable consequence of AXL activation, or vice versa. We therefore sought to induce EMT features in parental TAE684-sensitive cells and to determine whether this
effect would cause increased expression of AXL or, indeed, altered sensitivity to TAE684. Overexpression of FLAG-tagged _TWIST2_ (Figure 5a, left), the top EMT-associated gene that was
differentially expressed in the resistant cells (Figure 4b), led to significantly elevated vimentin and decreased cadherin levels (Figures 5b and c) in parental SH-SY5Y cells as compared
with vector-control transfected cells, as well as increased invasive capacity (Figure 5d). AXL expression, however, was not elevated in SH-SY5Y-TWIST2-expressing cells compared with control
cells (Figure 5e). Moreover, these cells remained as sensitive to TAE684 as untransfected or vector control-expressing cells (Figure 5f). In contrast, as shown previously, ectopic expression
of _AXL_ in parental TAE684-sensitive SH-SY5Y cells caused a twofold reduction in sensitivity to TAE684 (Figures 2e and f), although it did not alter _TWIST2_ expression levels (Figure 5e).
Overexpression of _AXL_ also led to a mesenchymal phenotype characterized by significant modulation of vimentin and cadherin expression (Figures 5b and c) and increased invasiveness (Figure
5d). Finally, simultaneous expression of both proteins in parental SH-SY5Y cells (Figure 5a, right) led to differential expression of vimentin and cadherin (Figures 5b and c), a highly
invasive phenotype (Figure 5d) and, importantly, more than threefold decrease in sensitivity to TAE684 (Figure 5f). Together, these observations suggest that, in our model, combined
expression of _AXL_ and _TWIST2_ leads to additive effects on EMT and drug resistance, whereas only AXL activation is associated with resistance to TAE684. AXL ACTIVATION IS FACILITATED BY
ELEVATED LEVELS OF THE CLEAVED FORM OF ITS LIGAND GAS6 The dependence of TAE684 resistance in NB cells on AXL activation led us to investigate the mechanism of _AXL_ upregulation in
SH-SY5Y-TR1 cells. Gene amplification was excluded at the outset through quantitative PCR analysis of genomic DNA (Supplementary Figure S3a). Sequencing of the _AXL_ promoter and coding
regions likewise failed to identify mutations within SH-SY5Y-TR1 cells that could account for elevated AXL expression (data not shown). Methylation-specific PCR and bisulfite sequencing
failed to reveal differential CpG hypomethylation as a mechanism of _AXL_ overexpression, as previously reported23 (data not shown). We also assessed the contribution of miR-34a and
miR-199a/b, the two acknowledged modulators of AXL expression,24 noting a decrease in miR-199b levels in the TAE684-resistant cells relative to the sensitive ones (Supplementary Figure S3b).
Forced expression of miR-199b in SH-SY5Y-TR1 cells, however, failed to induce downregulation of _AXL_ at either the mRNA (Supplementary Figure S3c) or protein (Supplementary Figure S3d)
level. Notably, no effect on pERK levels (Supplementary Figure S3d) was seen, suggesting that aberrant microRNA regulation was not a major determinant of the increased _AXL_ expression seen
in the TAE684-resistant NB cells. AXL receptor dimerization and activation through autophosphorylation has been reported to occur through binding of its physiological ligand GAS6 that
triggers a cascade of intracellular signaling events that culminate in cell proliferation and survival.25 _GAS6_ is normally expressed in two forms: the full-length 75 kDa protein and a
slightly larger protein, GAS6-SV (86 kDa), because of alternative splicing.26 This modification leads to the insertion of a 43-amino-acid sequence that contains a consensus cleavage site,
whose proteolytic cleavage results in a soluble 50 kDa product that is ultimately responsible for AXL receptor activation.26 We observed a marked increase in GAS6 protein levels in
TAE684-resistant SH-SY5Y-TR1 cells compared with parental cells, as well as significantly higher mRNA levels of the GAS6 splice variant, GAS6-SV (Figure 6a). Importantly, the same findings,
in conjunction with _AXL_ upregulation, were observed in SH-SY5Y cells that were made resistant to LDK378 (Figure 6b). Moreover, analysis of conditioned media from SH-SY5Y-TR1 cells revealed
an abundance of the cleaved active 50 kDa GAS6 fragment (Figure 6c). Hence, the presence of increased levels of GAS6, as well as that of its active secreted form in TAE684-resistant cells,
suggested that AXL activation in these cells resulted from upregulation of its ligand. To test this hypothesis, we depleted _GAS6_ in SH-SY5Y-TR1 cells through shRNA knockdown, noting a
resultant significant decrease in AXL levels and, more importantly, concomitant attenuation of ERK activation (Figure 6d). Moreover, conditioned media from SH-SY5Y-TR1 cells led to increased
levels of GAS6 and activated AXL proteins, as well as upregulated ERK signaling in TAE684-sensitive parental SH-SY5Y cells (Figure 6e). These cells became less sensitive to TAE684 while
showing increased sensitivity to R428 (Figure 6f). We also noted that _AXL_ mRNA levels in both parental and _AXL_-overexpressing SH-SY5Y cells did not increase after treatment with human
recombinant GAS6 (rGAS6), arguing against a positive feedback loop on AXL production (Supplementary Figure S4a). Rather, cells that were pretreated with rGAS6 showed higher AXL protein
levels in the presence of the protein synthesis inhibitor cycloheximide, suggesting that GAS6 upregulation led to AXL protein stabilization (Supplementary Figure S4b). Together, these
results suggest that AXL activation in SHSY5Y-TR1 cells is facilitated by increased production of soluble GAS6 via aberrant expression of cleavable GAS6-SV. TAE684-RESISTANT AXL-ACTIVATED
SH-SY5Y CELLS ARE SENSITIVE TO HSP90 INHIBITION It has been shown that ALK inhibitor-resistant non-small-cell lung cancer cell lines are susceptible to heat-shock protein 90 (HSP90)
inhibitors.27 Moreover, AXL is an acknowledged substrate of HSP90,28 prompting us to investigate the consequences of HSP90 inhibition in the context of NB cell resistance to TAE684. We
therefore tested the effects of the HSP90 inhibitors geldanamycin (17-AAG) and its semisynthetic derivative, retaspimycin hydrochloride (IPI-504),27 on TAE684-resistant NB cells. SH-SY5Y-TR1
cells showed a tenfold increase in sensitivity to HSP90 inhibition (Figure 7a). To identify the HSP90 targets whose inhibition led to such a striking response in TAE684-resistant cells, we
determined the phosphorylation status of several RTKs in SH-SY5Y-TR1 cells after treatment with IPI-504 (Figure 7b). Compared with DMSO-treated SH-SY5Y-TR1 cells, those treated with IPI-504
exhibited a loss of pAXL. EPHB2 phosphorylation was again seen to be increased in SH-SY5Y-TR1 cells (Figure 1c), but this effect was unchanged with IPI-504 treatment, suggesting that this
RTK most likely was not involved in TAE684-mediated resistance. The above results led us to ask whether the decreased phosphorylation of AXL upon HSP90 inhibition was coupled with
degradation and loss of total AXL in SH-SY5Y-TR1 cells. Treatment with IPI-504 led to a time-dependent reduction of total AXL levels in SH-SY5Y-TR1 cells (Figure 7c). Importantly, the
reduction in AXL levels on exposure to HSP90 inhibitor was accompanied by a concomitant decrease in pERK levels, again indicating that activated AXL signals at least partially through the
MAPK pathway in TAE684-resistant cells (Figure 7c). HSP90 functions as a molecular chaperone that stabilizes AXL through direct binding.28 To establish whether the interaction between AXL
and HSP90 in SH-SY5Y-TR1 cells was compromised by IPI-504, we coimmunoprecipitated HSP90 in resistant and parental cells before and after treatment with IPI-504 and analyzed AXL expression
by western blotting with an anti-AXL antibody. Although no AXL protein bound to HSP90 in SH-SY5Y cells, binding to HSP90 was observed in SH-SY5Y-TR1 cells, and this was markedly decreased
following treatment with IPI-504 (Figure 7d). Therefore, HSP90 inhibition in SH-SY5Y-TR1 cells leads to substantial reduction of AXL activity through diminished binding. Together, these
results support the hypothesis that activated AXL mediates resistance to ALK inhibition in ALKF1174L-driven NB cells and suggests a therapeutic strategy to overcome such resistance.
DISCUSSION Resistance to tyrosine kinase inhibitors of ALK has been described in multiple tumor types and can arise through different mechanisms.1 Here we show that in ALKF1174L-driven NB
cells, the development of resistance to TAE684 and its clinically available derivative, LDK378, is associated with _AXL_ overexpression and activation, as well as increased ERK signaling.
The resistant cells exhibit increased sensitivity to an AXL inhibitor in comparison with TAE684-sensitive parental cells, with concomitant downregulation of ERK signaling. Aberrantly
expressed AXL appears to be activated through increased levels of its ligand, GAS6, that also stabilizes the kinase. Resistance to TAE684 was associated with induction of an EMT phenotype.
Finally, TAE684-resistant cells were significantly more sensitive to HSP90 inhibition, at least partly through its impact on AXL binding. Our findings not only identify a molecular mechanism
of resistance to ALK inhibition in ALKF1174L-driven NB, but also suggest that effective AXL or HSP90 inhibitors, combined with a TAE684-derived ALK inhibitor, could provide a useful
strategy to overcome this complication. AXL is an RTK in the TAM kinase family whose members function as homeostatic regulators in adult tissues and play prominent roles in the nervous
system.15 When AXL is activated by its ligand GAS6,25 it also contributes to key physiological processes such as cell survival, proliferation and migration29 mainly through the MAPK and PI3K
signaling pathways.30, 31 _AXL_ overexpression has been reported in various cancers and its role in regulating the actin cytoskeleton links this kinase to tumor invasiveness and metastasis,
as shown in glioblastoma32 and breast cancer.33, 34 Moreover, _AXL_ overexpression has been implicated in resistance to both standard and targeted anticancer agents (mainly epidermal growth
factor receptor (EGFR) inhibitors) in various cancers, with or without accompanying EMT features.16, 17, 35, 36, 37 To our knowledge, this report provides the first evidence of AXL
overexpression and activation as an acquired mechanism of resistance to ALK inhibition in NB. We would stress that the findings presented here are restricted to human NB cells in which the
_ALK__F1174L_mutation is the principal if not the sole driver of tumorigenesis. This model accounts for ∼2% of all NB cases at diagnosis38 but does not necessarily apply to cases in which
_ALK__F1174L_ coexists with other major genetic aberrations such as _MYCN_ amplification. It will be important, therefore, to assess the significance of _AXL_ overexpression and activation
in cases of ALK-inhibitor resistance where the pathogenic role of _ALK_ mutations is less dominant. Interestingly, in a study by Duijkers _et al._,39 _AXL_ overexpression was observed in
established human NB cell lines that had not been exposed to targeted therapy, and its genetic depletion led to decreased cell migration and invasion, but not proliferation or downstream
signaling.39 The decreased growth kinetics and downregulated pERK after AXL depletion in the TAE684-resistant cells likely reflect their relatively higher dependence on increased AXL
activity. Whether AXL inhibition alone is sufficient to reverse resistance to TKIs remains unclear. Genetic and pharmacological inhibition of AXL has been shown to restore sensitivity to
erlotinib in _EGFR_-mutant lung cancer models with acquired erlotinib resistance, AXL activation and mesenchymal transition.17 However, in other studies, a functional role for AXL in both
erlotinib-resistant _EGFR_-positive or crizotinib-resistant _EML4-ALK_-positive lung cancer cells with _AXL_ overexpression and an EMT phenotype has been excluded.40, 41 Our results
demonstrate that AXL inhibition by itself only partly rescues TAE684 resistance, although it resensitizes these cells to TAE684, similar to results seen in head and neck cancer cells with
acquired erlotinib resistance.35 Thus, although AXL expression may contribute significantly to acquired resistance to TAE684, additional molecular mechanisms appear to be required for full
development of the resistance phenotype. Furthermore, the frequent association of _AXL_ upregulation with an EMT phenotype in acquired TKI resistance17, 35 raises the intriguing question of
whether AXL truly causes resistance or is merely a biomarker of EMT. In an analysis of multiple human cancer cell lines, in which elevated AXL was associated with a mesenchymal phenotype,
EMT-associated drug resistance was found to be independent of AXL function.41 However, our results indicate that both the acquisition of EMT features and AXL activation are required to
confer resistance to TAE684, although the relative contributions of these changes remain to be assessed. Overexpression of _TWIST2_, despite inducing an EMT phenotype, did not alter either
AXL expression or sensitivity to TAE684 in parental SH-SY5Y cells, whereas overexpression of _AXL_, although not affecting TWIST2 expression levels, led to an EMT phenotype and modest
resistance to TAE684. _TWIST2_, when combined with _AXL_ overexpression, led to a greater decrease in TAE684 sensitivity compared with ectopic expression of _AXL_ alone. These findings
suggest that the activation of AXL is independent of TWIST2 upregulation in TAE684-resistant cells, but can act cooperatively to enhance the resistant phenotype in response to ALK
inhibition. Of note, our results may be confounded by the fact that the gene expression patterns of ectopic overexpression of a single EMT-associated gene could differ from a phenotype that
develops over time. The mechanism of AXL activation appears to involve autocrine regulation through its ligand, GAS6. We observed increased levels of cleaved GAS6 protein in the supernatant
of TAE684-resistant cells as compared with the parental cells, suggesting that the active form of the ligand is secreted in order to sustain AXL activation and stabilization. Similarly, in
mesenchymal non-small-cell lung cancer cell lines with EMT features, resistance to erlotinib and upregulation of AXL was associated with markedly increased levels of GAS6.16, 17 Therefore,
cleaved soluble GAS6 could be exploited as a biomarker of resistance to ALK inhibition. Indeed, if validated in human samples from ALK inhibitor-treated patients, the ability to specifically
track cleaved GAS6 levels over time in the serum of patients would be a less invasive way to detect the early development of resistance. We observed that TAE684-resistant cells were highly
sensitive to HSP90 inhibition, partly due to depletion of AXL, leading to decreased downstream signaling. AXL was recently identified as an HSP90 substrate and was shown to be degraded in
the intracellular compartment by geldanamycin.42 The increased vulnerability of these cells may reflect the presence of other proteins, including other RTKs that contribute to cell
proliferation and survival, and by extension of the resistance phenotype, that are simultaneously disrupted with HSP90 inhibition. Our findings extend the currently emerging paradigm for the
design of HSP90 inhibition-based strategies, either alone or in combination with selective ALK targeting, in the management of ALK-driven resistant cancers. In ALK inhibitor-resistant
cells, the ERK pathway appeared to be a major signaling mechanism through which activated AXL contributed to resistance. Whereas the PI3K/AKT/mTOR pathway is primarily involved in ALK
downstream signaling in parental SH-SY5Y cells,5 pERK was upregulated in TAE684- and LDK378-resistant SH-SY5Y cells. Moreover, any alteration in AXL or GAS6 levels was closely correlated
with changes in ERK signaling in the resistant cells: genetic and pharmacological depletion of AXL led to decreased ERK phosphorylation, as did shRNA knockdown of _GAS6_, and HSP90
inhibitor-induced depletion of AXL. Although activated AXL could utilize multiple downstream signaling pathways, our observations highlight the ERK pathway as an essential signaling node
required to maintain cell survival in the face of targeted treatment. Indeed, two recent publications support the central role of ERK signaling in treatment resistance; first, MAPK pathway
mutations are often frequently found in relapsed NBs after chemotherapy,43 and, second, several RTK ligands can confer resistance to kinase inhibitors (including TAE684) by reactivation of
ERK in oncogene-addicted cancer cell lines.44 It is therefore reasonable to suggest that ERK inhibition with small molecules would be an effective strategy in therapy-resistant tumors or
even to prevent the emergence of resistance through this mechanism. Given the number of tumors that develop resistance to TKIs with upregulation of _AXL_, we propose that this mechanism is
common to any tumor targeted with these agents, and that it should be considered in patients who develop such resistance. Especially in NB, where clinical trials of ALK inhibitors alone or
in combination with standard chemotherapy agents are planned, it would be reasonable to assume that numerous instances of resistance will involve activation of AXL, and potentially the
development of EMT. Indeed, AXL or GAS6 expression could be used as biomarkers of resistance in tumors that have not yet acquired the EMT phenotype. As EMT is driven by transcription factors
that are currently undruggable, AXL activation represents an attractive target for inhibition in tumors resistant to ALK or other RTK inhibitors. As an alternative to an effective AXL
inhibitor, depletion of this RTK could also be readily achieved by HSP90 inhibition. MATERIALS AND METHODS CELL LINES AND REAGENTS The human NB cell line SK-N-SH and its derivative SH-SY5Y
were purchased from the American Type Culture Collection (Manassas, VA, USA) and their authenticity confirmed by genotyping. Cells were also confirmed to be mycoplasma negative. Parental and
resistant cells were grown in RPMI media supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. TAE684 and R428 were synthesized in-house in Dr Nathanael Gray’s laboratory
(Boston, MA, USA). 17-AAG, CHX and LDK378 were purchased from Selleck Chemicals (Houston, TX, USA), and IPI-504 was purchased from APExBIO (Houston, TX, USA). Recombinant human GAS6 (rGAS6)
was purchased from R&D Systems (885-GS-050; Minneapolis, MN, USA). CELL VIABILITY ASSAY Viability experiments were performed using the CellTiter-Glo Luminescent Cell Viability Assay
(G7573; Promega, Madison, WI, USA) according to the manufacturer’s instructions. All dose–response assays were performed in triplicate in 96-well plates and repeated at least three times.
The results, representing the mean±s.d. of three separate biological experiments, were plotted as a nonlinear regression curve fit using Graphpad Prism 6 software (La Jolla, CA, USA). The x
axis represents the log2 concentration of the indicated compound. WESTERN BLOTTING AND IMMUNOPRECIPITATION Cell lysates or conditioned media that were concentrated using Amicon Ultra 0.5 10K
Centrifugal Filters (EMD Millipore, Billerica, MA, USA) were prepared using standard protocols. The following antibodies were used: AXL (4566), pAXL (5724), ALK (3333), pALK (3341), AKT
(4691), pAKT (9271), ERK (4695), pERK (4377), VIM (5741), CDH1 (3195), tubulin (2128) and actin (4967) from Cell Signaling Technology (Danvers, MA, USA); GAS6 (sc-376087), HSP90 (sc-59577)
and phosphotyrosine (sc-81529) from Santa Cruz Biotechnology (Santa Cruz, CA, USA); Flag (F3165) from Sigma-Aldrich (Saint Louis, MO, USA); and AXL antibody for immunocytochemistry from
R&D Systems (AF154). PHOSPHO-RTK ARRAY ANALYSIS Cell lysate (500 μg) was incubated on a human phospho-RTK membrane array (ARY001B; R&D Systems) according to the manufacturer’s
instructions. Target proteins were captured with their respective antibodies. After washing, the proteins were incubated with a phosphotyrosine antibody conjugated to horseradish peroxidase
to allow the detection of captured phospho-RTKs. INVASION ASSAY A cell suspension containing 5 × 105 cells/ml in serum-free medium was added to the upper chamber of the invasion assembly
(ECM550; Chemicon International, Billerica, MA, USA) and 10% fetal bovine serum containing media added to the lower chamber to act as a chemoattractant. After incubation for 48 h,
non-migrating cells in the upper chamber were removed with cotton swabs, and cells that migrated to the lower surface of the filters were stained with crystal violet. The results were
quantified by counting and averaging three independent fields per condition, and represent the mean±s.d. of three separate biological experiments. LENTIVIRAL/RETROVIRAL TRANSDUCTION AND
TRANSIENT TRANSFECTION Lentiviral-based pLKO.1 shRNA constructs (_shAXL_, _shERK_ and _shGAS_6) were obtained from the RNAi Consortium of the Broad Institute (Cambridge, MA, USA). The
constructs were transfected into 293T cells with helper plasmids for virus production. Cells were then transduced with virus, followed by puromycin selection for at least 3 days. Stable
overexpression of the retroviral-based pMSCV vector containing _TWIST2_, _AXL_ or both genes was performed similarly. Transient knockdown of _AXL_ was performed with specific Silencer Select
siRNA (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. GAS6 siRNA pool was purchased from Dharmacon RNAi Technologies (Lafayette, CO, USA). GENE
EXPRESSION ANALYSIS Three biological replicates of total RNA were isolated from SH-SY5Y and SY5Y-TR1 cells using the RNeasy Mini kit (Qiagen, Valencia, CA, USA). Total RNA was hybridized to
GeneChip Human Genome U133 Plus 2.0 Arrays (Affymetrix, Santa Clara, CA, USA), according to the manufacturer’s instructions. The data obtained are accessible through the GEO accession number
GSE73292. Data analysis was performed using GenePattern software.45 GSEA was performed with the GSEA application,46 using log2 fold change to rank genes. IMMUNOCYTOCHEMISTRY 1 × 106 cells
were formalin fixed and immunocytochemistry performed based on established protocols.9 AXL antibody (R&D Systems) in a 1:1000 dilution was used to determine AXL expression. QUANTITATIVE
RT–PCR Total RNA was isolated using the RNeasy Mini kit (Qiagen), followed by RT–PCR with the ThermoScript RT–PCR system (Life Technologies). Quantitative PCR was carried out using the
QuantiFast SYBR Green PCR kit (Qiagen) in a 96-well plate format, and analyzed on an Applied Biosystems ViiA 7 Real-Time PCR System (Life Technologies). Each sample was run in triplicate and
normalized to actin as an internal control. Relative quantification was calculated according to the ΔΔCt relative quantification method. The results represent the mean±s.d. of three
separate biological experiments. Primer sequences are available upon request. SEQUENCE ANALYSIS The kinase domain of ALK and full-length AXL were amplified from complementary DNA of SH-SY5Y
and SY5Y-TR1 cells using the HotStar HiFidelity Polymerase Kit (Qiagen). The PCR products were cloned into the pGEM-T vector (Promega) and confirmed by sequencing. The GAS6 insertion
(GAS6-SV) was similarly amplified from the two cell lines, and the gel-purified PCR product confirmed by sequencing. STATISTICAL ANALYSIS Statistical significance for all comparisons between
two groups was determined with the two-sided Student’s _t_-test: *_P_<0.05, **_P_<0.01 and ***_P_<0.001. The effect of combining TAE684 and R428 was determined using the Bliss
additivity model.47 ACCESSION CODES ACCESSIONS GENE EXPRESSION OMNIBUS * GSE73292 REFERENCES * Gainor JF, Shaw AT . Emerging paradigms in the development of resistance to tyrosine kinase
inhibitors in lung cancer. _J Clin Oncol_ 2013; 31: 3987–3996. Article CAS PubMed PubMed Central Google Scholar * Mahon FX, Deininger MW, Schultheis B, Chabrol J, Reiffers J, Goldman JM
_et al_. Selection and characterization of BCR-ABL positive cell lines with differential sensitivity to the tyrosine kinase inhibitor STI571: diverse mechanisms of resistance. _Blood_ 2000;
96: 1070–1079. CAS PubMed Google Scholar * Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M _et al_. EGFR mutation and resistance of non-small-cell lung cancer to
gefitinib. _N Engl J Med_ 2005; 352: 786–792. Article CAS PubMed Google Scholar * Chen Y, Takita J, Choi YL, Kato M, Ohira M, Sanada M _et al_. Oncogenic mutations of ALK kinase in
neuroblastoma. _Nature_ 2008; 455: 971–974. Article CAS PubMed Google Scholar * George RE, Sanda T, Hanna M, Frohling S, Luther W 2nd, Zhang J _et al_. Activating mutations in ALK
provide a therapeutic target in neuroblastoma. _Nature_ 2008; 455: 975–978. Article CAS PubMed PubMed Central Google Scholar * Janoueix-Lerosey I, Lequin D, Brugieres L, Ribeiro A, de
Pontual L, Combaret V _et al_. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. _Nature_ 2008; 455: 967–970. Article CAS PubMed Google Scholar *
Mosse YP, Laudenslager M, Longo L, Cole KA, Wood A, Attiyeh EF _et al_. Identification of ALK as a major familial neuroblastoma predisposition gene. _Nature_ 2008; 455: 930–935. Article CAS
PubMed PubMed Central Google Scholar * De Brouwer S, De Preter K, Kumps C, Zabrocki P, Porcu M, Westerhout EM _et al_. Meta-analysis of neuroblastomas reveals a skewed ALK mutation
spectrum in tumors with MYCN amplification. _Clin Cancer Res_ 2010; 16: 4353–4362. Article CAS PubMed Google Scholar * Berry T, Luther W, Bhatnagar N, Jamin Y, Poon E, Sanda T _et al_.
The ALK(F1174L) mutation potentiates the oncogenic activity of MYCN in neuroblastoma. _Cancer Cell_ 2012; 22: 117–130. Article CAS PubMed PubMed Central Google Scholar * Sasaki T, Okuda
K, Zheng W, Butrynski J, Capelletti M, Wang L _et al_. The neuroblastoma-associated F1174L ALK mutation causes resistance to an ALK kinase inhibitor in ALK-translocated cancers. _Cancer
Res_ 2010; 70: 10038–10043. Article CAS PubMed PubMed Central Google Scholar * Sakamoto H, Tsukaguchi T, Hiroshima S, Kodama T, Kobayashi T, Fukami TA _et al_. CH5424802, a selective
ALK inhibitor capable of blocking the resistant gatekeeper mutant. _Cancer Cell_ 2011; 19: 679–690. Article CAS PubMed Google Scholar * Galkin AV, Melnick JS, Kim S, Hood TL, Li N, Li L
_et al_. Identification of NVP-TAE684, a potent, selective, and efficacious inhibitor of NPM-ALK. _Proc Natl Acad Sci USA_ 2007; 104: 270–275. Article CAS PubMed Google Scholar * Chen J,
Jiang C, Wang S . LDK378: a promising anaplastic lymphoma kinase (ALK) inhibitor. _J Med Chem_ 2013; 56: 5673–5674. Article CAS PubMed Google Scholar * Heuckmann JM, Holzel M, Sos ML,
Heynck S, Balke-Want H, Koker M _et al_. ALK mutations conferring differential resistance to structurally diverse ALK inhibitors. _Clin Cancer Res_ 2011; 17: 7394–7401. Article CAS PubMed
PubMed Central Google Scholar * Lemke G . Biology of the TAM receptors. _Cold Spring Harb Perspect Biol_ 2013; 5: a009076. Article PubMed PubMed Central Google Scholar * Byers LA,
Diao L, Wang J, Saintigny P, Girard L, Peyton M _et al_. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a
therapeutic target for overcoming EGFR inhibitor resistance. _Clin Cancer Res_ 2013; 19: 279–290. Article CAS PubMed Google Scholar * Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise
T _et al_. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. _Nat Genet_ 2012; 44: 852–860. Article CAS PubMed PubMed Central Google Scholar *
Holland SJ, Pan A, Franci C, Hu Y, Chang B, Li W _et al_. R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast
cancer. _Cancer Res_ 2010; 70: 1544–1554. Article CAS PubMed Google Scholar * Anastassiou D, Rumjantseva V, Cheng W, Huang J, Canoll PD, Yamashiro DJ _et al_. Human cancer cells express
Slug-based epithelial-mesenchymal transition gene expression signature obtained _in vivo_. _BMC Cancer_ 2011; 11: 529. Article CAS PubMed PubMed Central Google Scholar * Blick T, Hugo
H, Widodo E, Waltham M, Pinto C, Mani SA _et al_. Epithelial mesenchymal transition traits in human breast cancer cell lines parallel the CD44(hi/)CD24 (lo/-) stem cell phenotype in human
breast cancer. _J Mammary Gland Biol Neoplasia_ 2010; 15: 235–252. Article PubMed Google Scholar * Taube JH, Herschkowitz JI, Komurov K, Zhou AY, Gupta S, Yang J _et al_. Core
epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. _Proc Natl Acad Sci USA_ 2010; 107:
15449–15454. Article CAS PubMed PubMed Central Google Scholar * Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P _et al_. Genotypic and histological evolution
of lung cancers acquiring resistance to EGFR inhibitors. _Sci Transl Med_ 2011; 3: 75ra26. Article PubMed PubMed Central Google Scholar * Mudduluru G, Allgayer H . The human receptor
tyrosine kinase Axl gene—promoter characterization and regulation of constitutive expression by Sp1, Sp3 and CpG methylation. _Biosci Rep_ 2008; 28: 161–176. CAS PubMed Google Scholar *
Mudduluru G, Ceppi P, Kumarswamy R, Scagliotti GV, Papotti M, Allgayer H . Regulation of Axl receptor tyrosine kinase expression by miR-34a and miR-199a/b in solid cancer. _Oncogene_ 2011;
30: 2888–2899. Article CAS PubMed Google Scholar * Mark MR, Chen J, Hammonds RG, Sadick M, Godowsk PJ . Characterization of Gas6, a member of the superfamily of G domain-containing
proteins, as a ligand for Rse and Axl. _J Biol Chem_ 1996; 271: 9785–9789. Article CAS PubMed Google Scholar * Goruppi S, Yamane H, Marcandalli P, Garcia A, Clogston C, Gostissa M _et
al_. The product of a gas6 splice variant allows the release of the domain responsible for Axl tyrosine kinase receptor activation. _FEBS Lett_ 1997; 415: 59–63. Article CAS PubMed Google
Scholar * Normant E, Paez G, West KA, Lim AR, Slocum KL, Tunkey C _et al_. The Hsp90 inhibitor IPI-504 rapidly lowers EML4-ALK levels and induces tumor regression in ALK-driven NSCLC
models. _Oncogene_ 2011; 30: 2581–2586. Article CAS PubMed Google Scholar * Wu Z, Moghaddas Gholami A, Kuster B . Systematic identification of the HSP90 candidate regulated proteome.
_Mol Cell Proteomics_ 2012; 11: M111 016675. Article PubMed PubMed Central Google Scholar * Korshunov VA . Axl-dependent signalling: a clinical update. _Clin Sci (Lond)_ 2012; 122:
361–368. Article CAS Google Scholar * Stenhoff J, Dahlback B, Hafizi S . Vitamin K-dependent Gas6 activates ERK kinase and stimulates growth of cardiac fibroblasts. _Biochem Biophys Res
Commun_ 2004; 319: 871–878. Article CAS PubMed Google Scholar * Fridell YW, Jin Y, Quilliam LA, Burchert A, McCloskey P, Spizz G _et al_. Differential activation of the
Ras/extracellular-signal-regulated protein kinase pathway is responsible for the biological consequences induced by the Axl receptor tyrosine kinase. _Mol Cell Biol_ 1996; 16: 135–145.
Article CAS PubMed PubMed Central Google Scholar * Vajkoczy P, Knyazev P, Kunkel A, Capelle HH, Behrndt S, von Tengg-Kobligk H _et al_. Dominant-negative inhibition of the Axl receptor
tyrosine kinase suppresses brain tumor cell growth and invasion and prolongs survival. _Proc Natl Acad Sci USA_ 2006; 103: 5799–5804. Article CAS PubMed PubMed Central Google Scholar *
Gjerdrum C, Tiron C, Hoiby T, Stefansson I, Haugen H, Sandal T _et al_. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient
survival. _Proc Natl Acad Sci USA_ 2010; 107: 1124–1129. Article CAS PubMed Google Scholar * Vuoriluoto K, Haugen H, Kiviluoto S, Mpindi JP, Nevo J, Gjerdrum C _et al_. Vimentin
regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. _Oncogene_ 2011; 30: 1436–1448. Article CAS PubMed Google Scholar * Giles
KM, Kalinowski FC, Candy PA, Epis MR, Zhang PM, Redfern AD _et al_. Axl mediates acquired resistance of head and neck cancer cells to the epidermal growth factor receptor inhibitor
erlotinib. _Mol Cancer Ther_ 2013; 12: 2541–2558. Article CAS PubMed Google Scholar * Hong J, Peng D, Chen Z, Sehdev V, Belkhiri A . ABL regulation by AXL promotes cisplatin resistance
in esophageal cancer. _Cancer Res_ 2013; 73: 331–340. Article CAS PubMed Google Scholar * Liu L, Greger J, Shi H, Liu Y, Greshock J, Annan R _et al_. Novel mechanism of lapatinib
resistance in HER2-positive breast tumor cells: activation of AXL. _Cancer Res_ 2009; 69: 6871–6878. Article CAS PubMed Google Scholar * Bresler SC, Weiser DA, Huwe PJ, Park JH, Krytska
K, Ryles H _et al_. ALK mutations confer differential oncogenic activation and sensitivity to ALK inhibition therapy in neuroblastoma. _Cancer Cell_ 2014; 26: 682–694. Article CAS PubMed
PubMed Central Google Scholar * Duijkers FA, Meijerink JP, Pieters R, van Noesel MM . Downregulation of Axl in non-MYCN amplified neuroblastoma cell lines reduces migration. _Gene_ 2013;
521: 62–68. Article CAS PubMed Google Scholar * Kim HR, Kim WS, Choi YJ, Choi CM, Rho JK, Lee JC . Epithelial-mesenchymal transition leads to crizotinib resistance in H2228 lung cancer
cells with EML4-ALK translocation. _Mol Oncol_ 2013; 7: 1093–1102. Article CAS PubMed PubMed Central Google Scholar * Wilson C, Ye X, Pham T, Lin E, Chan S, McNamara E _et al_. AXL
inhibition sensitizes mesenchymal cancer cells to antimitotic drugs. _Cancer Res_ 2014; 74: 5878–5890. Article CAS PubMed Google Scholar * Krishnamoorthy GP, Guida T, Alfano L, Avilla E,
Santoro M, Carlomagno F _et al_. Molecular mechanism of 17-allylamino-17-demethoxygeldanamycin (17-AAG)-induced AXL receptor tyrosine kinase degradation. _J Biol Chem_ 2013; 288:
17481–17494. Article CAS PubMed PubMed Central Google Scholar * Eleveld TF, Oldridge DA, Bernard V, Koster J, Daage LC, Diskin SJ _et al_. Relapsed neuroblastomas show frequent RAS-MAPK
pathway mutations. _Nat Genet_ 2015; 47: 864–871. Article CAS PubMed PubMed Central Google Scholar * Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E _et al_. Widespread
potential for growth-factor-driven resistance to anticancer kinase inhibitors. _Nature_ 2012; 487: 505–509. Article CAS PubMed PubMed Central Google Scholar * Reich M, Liefeld T, Gould
J, Lerner J, Tamayo P, Mesirov JP . GenePattern 2.0. _Nat Genet_ 2006; 38: 500–501. Article CAS PubMed Google Scholar * Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL,
Gillette MA _et al_. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. _Proc Natl Acad Sci USA_ 2005; 102: 15545–15550. Article CAS
PubMed PubMed Central Google Scholar * Greco WR, Bravo G, Parsons JC . The search for synergy: a critical review from a response surface perspective. _Pharmacol Rev_ 1995; 47: 331–385.
CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS This study was supported by NIH/NCI R01 CA148688 (to REG), Friends for Life Neuroblastoma Fellowship (to DND) and a Ruth L
Kirschstein NRSA F32CA183566 (to NFM). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pediatric Hematology/Oncology, Dana-Farber Cancer Institute and Boston Children’s Hospital,
Boston, MA, USA D N Debruyne, N Bhatnagar, B Sharma, W Luther, N F Moore & R E George * Department of Pediatric Oncology, Memorial Sloan-Kettering Cancer Center, New York, NY, USA N-K
Cheung * Department of Cancer Biology, Dana-Farber Cancer Institute, Boston, MA, USA N S Gray * Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston,
MA, USA N S Gray * Department of Pediatrics, Harvard Medical School, Boston, MA, USA R E George Authors * D N Debruyne View author publications You can also search for this author inPubMed
Google Scholar * N Bhatnagar View author publications You can also search for this author inPubMed Google Scholar * B Sharma View author publications You can also search for this author
inPubMed Google Scholar * W Luther View author publications You can also search for this author inPubMed Google Scholar * N F Moore View author publications You can also search for this
author inPubMed Google Scholar * N-K Cheung View author publications You can also search for this author inPubMed Google Scholar * N S Gray View author publications You can also search for
this author inPubMed Google Scholar * R E George View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to R E George.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION Supplementary Information accompanies this paper on the Oncogene website
SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE LEGENDS (PDF 9 KB) SUPPLEMENTARY FIGURE S1 (JPG 154 KB) SUPPLEMENTARY FIGURE S2 (JPG 233 KB) SUPPLEMENTARY FIGURE S3 (JPG 319 KB) SUPPLEMENTARY
FIGURE S4 (JPG 185 KB) RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article
are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to
obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Debruyne, D., Bhatnagar, N., Sharma, B. _et al._ ALK inhibitor resistance in ALKF1174L-driven neuroblastoma is associated with AXL activation and induction of EMT.
_Oncogene_ 35, 3681–3691 (2016). https://doi.org/10.1038/onc.2015.434 Download citation * Received: 18 February 2015 * Revised: 15 September 2015 * Accepted: 11 October 2015 * Published: 30
November 2015 * Issue Date: 14 July 2016 * DOI: https://doi.org/10.1038/onc.2015.434 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
