- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
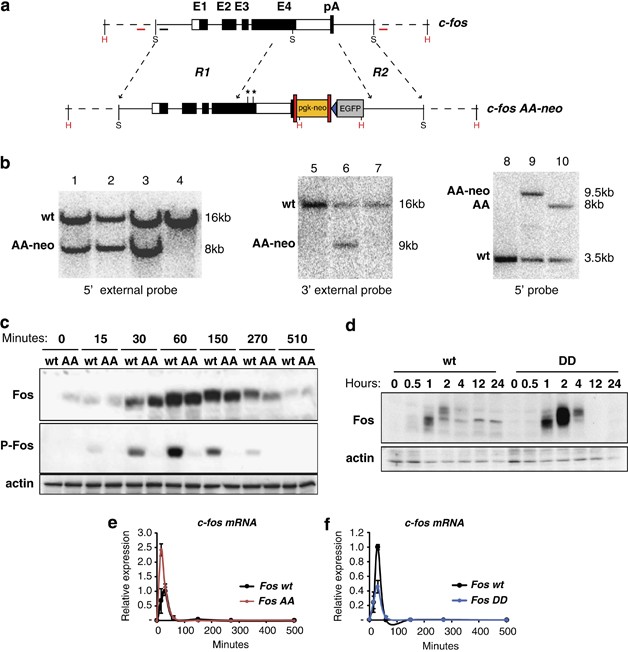
ABSTRACT Mice lacking _c-fos_ develop osteopetrosis due to a block in osteoclast differentiation. Carboxy-terminal phosphorylation of Fos on serine 374 by ERK1/2 and serine 362 by RSK1/2
regulates Fos stability and transactivation potential _in vitro_. To assess the physiological relevance of Fos phosphorylation _in vivo_, serine 362 and/or serine 374 was replaced by alanine
(Fos362A, Fos374A and FosAA) or by phospho-mimetic aspartic acid (FosDD). Homozygous mutants were healthy and skeletogenesis was largely unaffected. Fos C-terminal phosphorylation,
predominantly on serine 374, was found important for osteoclast differentiation _in vitro_ and affected lipopolysaccharide (LPS)-induced cytokine response _in vitro_ and _in vivo_.
Importantly, skin papilloma development was delayed in FosAA, Fos362A and _Rsk2_-deficient mice, accelerated in FosDD mice and unaffected in Fos374A mutants. Furthermore, the related Fos
protein and putative RSK2 target Fra1 failed to substitute for Fos in papilloma development. This indicates that phosphorylation of serines 362 and 374 exerts context-dependent roles in
modulating Fos activity _in vivo_. Inhibition of Fos C-terminal phosphorylation on serine 362 by targeting RSK2 might be of therapeutic relevance for skin tumours. Access through your
institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 50 print
issues and online access $259.00 per year only $5.18 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to
local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT
BEING VIEWED BY OTHERS MEF2C REGULATES OSTEOCLASTOGENESIS AND PATHOLOGIC BONE RESORPTION VIA C-FOS Article Open access 11 January 2021 WNT SIGNALING AND LOXL2 PROMOTE AGGRESSIVE OSTEOSARCOMA
Article Open access 20 July 2020 INHIBITION OF CDK5 INCREASES OSTEOBLAST DIFFERENTIATION AND BONE MASS AND IMPROVES FRACTURE HEALING Article Open access 06 April 2022 REFERENCES * Bakiri L,
Takada Y, Radolf M, Eferl R, Yaniv M, Wagner EF _et al_. (2007). Role of heterodimerization of c-Fos and Fra1 proteins in osteoclast differentiation. _Bone_ 40: 867–875. Article CAS
PubMed Google Scholar * Barber JR, Verma IM . (1987). Modification of fos proteins: phosphorylation of c-fos, but not v-fos, is stimulated by 12-tetradecanoyl-phorbol-13-acetate and serum.
_Mol Cell Biol_ 7: 2201–2211. Article CAS PubMed PubMed Central Google Scholar * Basbous J, Chalbos D, Hipskind R, Jariel-Encontre I, Piechaczyk M . (2007). Ubiquitin-independent
proteasomal degradation of Fra-1 is antagonized by Erk1/2 pathway-mediated phosphorylation of a unique C-terminal destabilizer. _Mol Cell Biol_ 27: 3936–3950. Article CAS PubMed PubMed
Central Google Scholar * Casalino L, Bakiri L, Talotta F, Weitzman JB, Fusco A, Yaniv M _et al_. (2007). Fra-1 promotes growth and survival in RAS-transformed thyroid cells by controlling
cyclin A transcription. _EMBO J_ 26: 1878–1890. Article CAS PubMed PubMed Central Google Scholar * Chen RH, Abate C, Blenis J . (1993). Phosphorylation of the c-Fos transrepression
domain by mitogen-activated protein kinase and 90-kDa ribosomal S6 kinase. _Proc Natl Acad Sci USA_ 90: 10952–10956. Article CAS PubMed PubMed Central Google Scholar * Chen RH, Juo PC,
Curran T, Blenis J . (1996). Phosphorylation of c-Fos at the C-terminus enhances its transforming activity. _Oncogene_ 12: 1493–1502. CAS PubMed Google Scholar * Clausen BE, Burkhardt C,
Reith W, Renkawitz R, Forster I . (1999). Conditional gene targeting in macrophages and granulocytes using LysMcre mice. _Transgenic Res_ 8: 265–277. Article CAS PubMed Google Scholar *
Curran T, Miller AD, Zokas L, Verma IM . (1984). Viral and cellular Fos proteins: a comparative analysis. _Cell_ 36: 259–268. Article CAS PubMed Google Scholar * David JP, Mehic D,
Bakiri L, Schilling AF, Mandic V, Priemel M _et al_. (2005). Essential role of RSK2 in c-Fos-dependent osteosarcoma development. _J Clin Invest_ 115: 664–672. Article CAS PubMed PubMed
Central Google Scholar * Deng T, Karin M . (1994). c-Fos transcriptional activity stimulated by H-ras-activated protein kinase distinct from JNK and ERK. _Nature_ 371: 171–175. Article
CAS PubMed Google Scholar * Doehn U, Hauge C, Frank SR, Jensen CJ, Duda K, Nielsen JV _et al_. (2009). RSK is a principal effector of the RAS-ERK pathway for eliciting a coordinate
promotile/invasive gene program and phenotype in epithelial cells. _Mol Cell_ 35: 511–522. Article CAS PubMed PubMed Central Google Scholar * Durchdewald M, Guinea-Viniegra J, Haag D,
Riehl A, Lichter P, Hahn M _et al_. (2008). Podoplanin is a novel Fos target gene in skin carcinogenesis. _Cancer Res_ 68: 6877–6883. Article CAS PubMed Google Scholar * Dymecki SM .
(1996). Flp recombinase promotes site-specific DNA recombination in embryonic stem cells and transgenic mice. _Proc Natl Acad Sci USA_ 93: 6191–6196. Article CAS PubMed PubMed Central
Google Scholar * Eferl R, Wagner EF . (2003). AP-1: a double-edged sword in tumorigenesis. _Nat Rev Cancer_ 3: 859–868. Article CAS PubMed Google Scholar * Ferrara P, Andermarcher E,
Bossis G, Acquaviva C, Brockly F, Jariel-Encontre I _et al_. (2003). The structural determinants responsible for c-Fos protein proteasomal degradation differ according to the conditions of
expression. _Oncogene_ 22: 1461–1474. Article CAS PubMed Google Scholar * Fleischmann A, Hafezi F, Elliott C, Reme CE, Ruther U, Wagner EF . (2000). Fra-1 replaces c-Fos-dependent
functions in mice. _Genes Dev_ 14: 2695–2700. Article CAS PubMed PubMed Central Google Scholar * Fleischmann A, Hvalby O, Jensen V, Strekalova T, Zacher C, Layer LE _et al_. (2003a).
Impaired long-term memory and NR2A-type NMDA receptor-dependent synaptic plasticity in mice lacking c-Fos in the CNS. _J Neurosci_ 23: 9116–9122. Article CAS PubMed PubMed Central Google
Scholar * Fleischmann A, Jochum W, Eferl R, Witowsky J, Wagner EF . (2003b). Rhabdomyosarcoma development in mice lacking Trp53 and Fos: tumor suppression by the Fos protooncogene. _Cancer
Cell_ 4: 477–482. Article CAS PubMed Google Scholar * Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G . (2005). The molecular clock mediates leptin-regulated bone formation. _Cell_
122: 803–815. Article CAS PubMed Google Scholar * Gius D, Cao XM, Rauscher III FJ, Cohen DR, Curran T, Sukhatme VP . (1990). Transcriptional activation and repression by Fos are
independent functions: the C terminus represses immediate-early gene expression via CArG elements. _Mol Cell Biol_ 10: 4243–4255. Article CAS PubMed PubMed Central Google Scholar *
Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA _et al_. (1994). c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. _Science_
266: 443–448. Article CAS PubMed Google Scholar * Hess J, Angel P, Schorpp-Kistner M . (2004). AP-1 subunits: quarrel and harmony among siblings. _J Cell Sci_ 117: 5965–5973. Article
CAS PubMed Google Scholar * Koga K, Takaesu G, Yoshida R, Nakaya M, Kobayashi T, Kinjyo I _et al_. (2009). Cyclic adenosine monophosphate suppresses the transcription of proinflammatory
cytokines via the phosphorylated c-Fos protein. _Immunity_ 30: 372–383. Article CAS PubMed Google Scholar * Monje P, Marinissen MJ, Gutkind JS . (2003). Phosphorylation of the
carboxyl-terminal transactivation domain of c-Fos by extracellular signal-regulated kinase mediates the transcriptional activation of AP-1 and cellular transformation induced by
platelet-derived growth factor. _Mol Cell Biol_ 23: 7030–7043. Article CAS PubMed PubMed Central Google Scholar * Murphy LO, Blenis J . (2006). MAPK signal specificity: the right place
at the right time. _Trends Biochem Sci_ 31: 268–275. Article CAS PubMed Google Scholar * Murphy LO, Smith S, Chen RH, Fingar DC, Blenis J . (2002). Molecular interpretation of ERK signal
duration by immediate early gene products. _Nat Cell Biol_ 4: 556–564. Article CAS PubMed Google Scholar * Nakakuki T, Birtwistle MR, Saeki Y, Yumoto N, Ide K, Nagashima T _et al_.
(2010). Ligand-specific c-Fos expression emerges from the spatiotemporal control of ErbB network dynamics. _Cell_ 141: 884–896. Article CAS PubMed PubMed Central Google Scholar * Ofir
R, Dwarki VJ, Rashid D, Verma IM . (1990). Phosphorylation of the C terminus of Fos protein is required for transcriptional transrepression of the c-fos promoter. _Nature_ 348: 80–82.
Article CAS PubMed Google Scholar * Okazaki K, Sagata N . (1995). The Mos/MAP kinase pathway stabilizes c-Fos by phosphorylation and augments its transforming activity in NIH 3T3 cells.
_EMBO J_ 14: 5048–5059. Article CAS PubMed PubMed Central Google Scholar * Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ _et al_. (1987). Bone histomorphometry:
standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. _J Bone Miner Res_ 2: 595–610. Article CAS PubMed Google Scholar *
Pellegrino MJ, Stork PJ . (2006). Sustained activation of extracellular signal-regulated kinase by nerve growth factor regulates c-fos protein stabilization and transactivation in PC12
cells. _J Neurochem_ 99: 1480–1493. Article CAS PubMed Google Scholar * Ray N, Kuwahara M, Takada Y, Maruyama K, Kawaguchi T, Tsubone H _et al_. (2006). c-Fos suppresses systemic
inflammatory response to endotoxin. _Int Immunol_ 18: 671–677. Article CAS PubMed Google Scholar * Saez E, Rutberg SE, Mueller E, Oppenheim H, Smoluk J, Yuspa SH _et al_. (1995). c-fos
is required for malignant progression of skin tumors. _Cell_ 82: 721–732. Article CAS PubMed Google Scholar * Sasaki T, Kojima H, Kishimoto R, Ikeda A, Kunimoto H, Nakajima K . (2006).
Spatiotemporal regulation of c-Fos by ERK5 and the E3 ubiquitin ligase UBR1, and its biological role. _Mol Cell_ 24: 63–75. Article CAS PubMed Google Scholar * Sassone-Corsi P, Sisson
JC, Verma IM . (1988). Transcriptional autoregulation of the proto-oncogene fos. _Nature_ 334: 314–319. Article CAS PubMed Google Scholar * Shin S, Dimitri CA, Yoon SO, Dowdle W, Blenis
J . (2010). ERK2 but not ERK1 induces epithelial-to-mesenchymal transformation via DEF motif-dependent signaling events. _Mol Cell_ 38: 114–127. Article CAS PubMed PubMed Central Google
Scholar * Sibilia M, Fleischmann A, Behrens A, Stingl L, Carroll J, Watt FM _et al_. (2000). The EGF receptor provides an essential survival signal for SOS-dependent skin tumor development.
_Cell_ 102: 211–220. Article CAS PubMed Google Scholar * Takada Y, Ray N, Ikeda E, Kawaguchi T, Kuwahara M, Wagner EF _et al_. (2010). Fos proteins suppress dextran sulfate
sodium-induced colitis through inhibition of NF-kappaB. _J Immunol_ 184: 1014–1021. Article CAS PubMed Google Scholar * Tratner I, Ofir R, Verma IM . (1992). Alteration of a cyclic
AMP-dependent protein kinase phosphorylation site in the c-Fos protein augments its transforming potential. _Mol Cell Biol_ 12: 998–1006. Article CAS PubMed PubMed Central Google Scholar
* Wang ZQ, Liang J, Schellander K, Wagner EF, Grigoriadis AE . (1995). c-fos-induced osteosarcoma formation in transgenic mice: cooperativity with c-jun and the role of endogenous c-fos.
_Cancer Res_ 55: 6244–6251. CAS PubMed Google Scholar * Wang ZQ, Ovitt C, Grigoriadis AE, Mohle-Steinlein U, Ruther U, Wagner EF . (1992). Bone and haematopoietic defects in mice lacking
c-fos. _Nature_ 360: 741–745. Article CAS PubMed Google Scholar * Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T _et al_. (2004). ATF4 is a substrate of RSK2 and an
essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. _Cell_ 117: 387–398. Article CAS PubMed Google Scholar * Zenz R, Scheuch H, Martin P, Frank C, Eferl R,
Kenner L _et al_. (2003). c-Jun regulates eyelid closure and skin tumor development through EGFR signaling. _Dev Cell_ 4: 879–889. Article CAS PubMed Google Scholar Download references
ACKNOWLEDGEMENTS We are very grateful to Drs K Matsuo, Y Takada and R Khokha for critical comments and helpful suggestions, Drs F Mulero and RP Marshall for help with micro-computed
tomography, U Moehle-Steinlein, E Andres, HC Theussl and V Komnenovic for technical assistance. The project was initiated at the IMP, which is funded by Boehringer Ingelheim (BI). EFW is
funded by the BBVA-Foundation and by an ERC advanced Grant. Part of this work was funded by an EMBO postdoctoral fellowship to LB and the NoE Cells into Organs (LSHM-CT-2003-504468) program
of the European Community. AUTHOR INFORMATION Author notes * R Zenz Present address: 5Current address: Ludwig Boltzmann Institute for Cancer Research, Vienna, Austria., * M O Reschke:
Currrent address: Division of Genetics, Beth Israel Deaconess Medical Center, Boston, MA, USA. AUTHORS AND AFFILIATIONS * Genes, Development and Disease Group, F-BBVA Cancer Cell Biology
programme, National Cancer Research Centre (CNIO), Madrid, Spain L Bakiri, M O Reschke, H A Gefroh, M H Idarraga, K Polzer, R Zenz, G Schett & E F Wagner * Research Institute of
Molecular Pathology (IMP), Vienna, Austria M H Idarraga * Department of Internal Medicine 3, University of Erlangen-Nurenberg, Erlangen, Germany K Polzer & G Schett Authors * L Bakiri
View author publications You can also search for this author inPubMed Google Scholar * M O Reschke View author publications You can also search for this author inPubMed Google Scholar * H A
Gefroh View author publications You can also search for this author inPubMed Google Scholar * M H Idarraga View author publications You can also search for this author inPubMed Google
Scholar * K Polzer View author publications You can also search for this author inPubMed Google Scholar * R Zenz View author publications You can also search for this author inPubMed Google
Scholar * G Schett View author publications You can also search for this author inPubMed Google Scholar * E F Wagner View author publications You can also search for this author inPubMed
Google Scholar CORRESPONDING AUTHOR Correspondence to E F Wagner. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION Supplementary
Information accompanies the paper on the Oncogene website SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES 1–5 (PDF 2252 KB) SUPPLEMENTARY FIGURE LEGENDS (DOC 27 KB) RIGHTS AND PERMISSIONS
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Bakiri, L., Reschke, M., Gefroh, H. _et al._ Functions of Fos phosphorylation in bone homeostasis, cytokine response and
tumourigenesis. _Oncogene_ 30, 1506–1517 (2011). https://doi.org/10.1038/onc.2010.542 Download citation * Received: 16 August 2010 * Revised: 20 September 2010 * Accepted: 10 October 2010 *
Published: 29 November 2010 * Issue Date: 31 March 2011 * DOI: https://doi.org/10.1038/onc.2010.542 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
KEYWORDS * AP-1 * Fos * knock-in * mouse * phosphorylation