- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
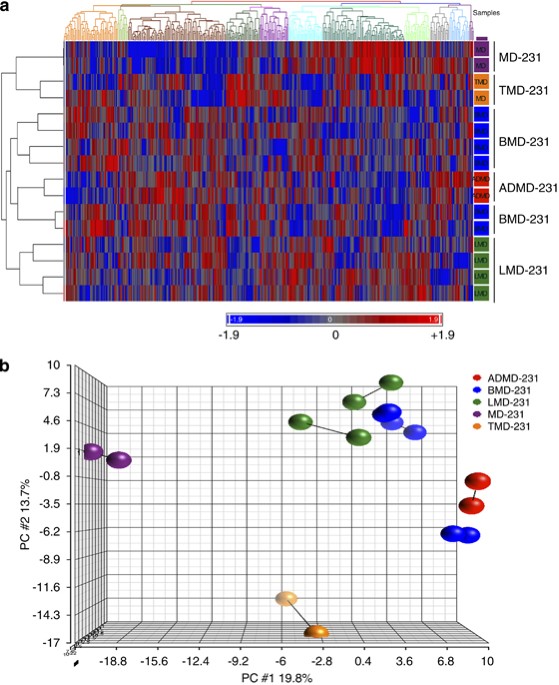
Metastasis in breast cancer carries a disproportionately worse prognosis than localized primary disease. To identify microRNAs (miRNA) involved in metastasis, the expression of 254 miRNAs
was measured across the following cell lines using microarray analysis: MDA-MB-231 breast cancer cells, cells that grew as a tumor in the mammary fat pad of nude mice (TMD-231), metastatic
disease to the lungs (LMD-231), bone (BMD-231) and adrenal gland (ADMD-231). A brain-seeking variant of this cell line (231-BR) was used additionally in validation studies. Twenty miRNAs
were upregulated and seven were downregulated in metastatic cancer cells compared with TMD-231 cells. The expression of the tumor suppressor miRNAs let-7 and miR-22 was consistently
downregulated in metastatic cancer cells. These metastatic cells expressed higher levels of putative/proven miR-22 target oncogenes ERBB3, CDC25C and EVI-1. Introduction of miR-22 into
cancer cells reduced the levels of ERBB3 and EVI-1 as well as phospho-AKT, an EVI-1 downstream target. The miR-22 primary transcript is located in the 5′-untranslated region of an open
reading frame C17orf91, and the promoter/enhancer of C17orf91 drives miR-22 expression. We observed elevated C17orf91 expression in non-basal subtype compared with basal subtype breast
cancers. In contrast, elevated expression of EVI-1 was observed in basal subtype and was associated with poor outcome in estrogen receptor-negative breast cancer patients. These results
suggest that metastatic cancer cells increase specific oncogenic signaling proteins through downregulation of miRNAs. Identifying such metastasis-specific oncogenic pathways may help to
manipulate tumor behavior and aid in the design of more effective targeted therapies.
This work is supported by Indiana University Simon Cancer Center Pilot grant and by Komen for Cure grant BCTR0601111 to HN. JBP and RMB are supported by National Institutes of Health
Training Grants T32 DK07519 and T32 CA111198, respectively. HN is Marian J Morrison Professor of Breast Cancer Research.
J B Patel and H N Appaiah: These authors contributed equally to this work.
Department of Surgery, Indiana University School of Medicine, Indianapolis, IN, USA
J B Patel, H N Appaiah, R M Burnett, P Bhat-Nakshatri & H Nakshatri
Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA
Department of Pathology, Indiana University School of Medicine, Indianapolis, IN, USA
Department of Cancer Biology, Vanderbilt-Ingram Cancer Center, Vanderbilt University Medical School, Nashville, TN, USA
Department of Cell and Developmental Biology, Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, NC, USA
Laboratory of Molecular Pharmacology, Center for Cancer Research National Cancer Institute, Bethesda, MD, USA
Department of Biochemistry and Molecular Biology, Indiana University School of Medicine, Indianapolis, IN, USA
Anyone you share the following link with will be able to read this content: