- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
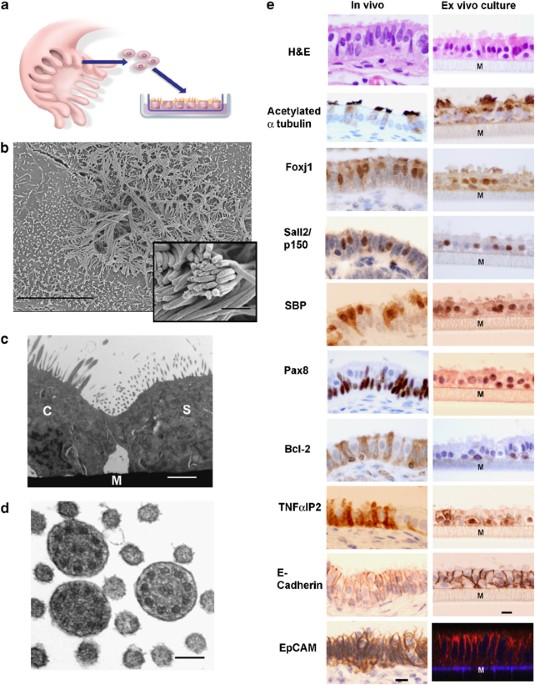
Recent studies suggest that some serous ovarian carcinomas (SOCs) arise from the fallopian tube (FT) epithelium rather than the ovarian surface epithelium. This hypothesis places emphasis on
the FT secretory epithelial cell as a cell-of-origin. Herein, we report the development of a novel ex vivo primary human FT epithelium culture system that faithfully recapitulates the in
vivo epithelium, as shown by morphological, ultrastructural and immunophenotypic analyses. Mass spectrometry-based proteomics reveal that these cultures secrete proteins previously
identified as biomarkers for ovarian cancer. We also use this culture system to study the response of the FT epithelium to genotoxic stress and find that the secretory cells exhibit a
distinct response to DNA damage when compared with neighboring ciliated cells. The secretory cells show a limited ability to resolve the damage over time, potentially leaving them more
susceptible to accumulation of additional mutagenic injury. This divergent response is confirmed with in situ studies using tissue samples, further supporting the use of this ex vivo culture
system to investigate FT epithelial pathobiology. We anticipate that this novel culture system will facilitate the study of SOC pathogenesis, and propose that similar culture systems could
be developed for other organ site-specific epithelia.
We thank Phil Karp, Thomas Moninger and Drs Paola Vermeer and Joseph Zabner (University of Iowa) for their enthusiastic support and assistance in establishing the FT ex vivo culture system,
Drs Steve Cannistra, Glenn Dranoff and David Livingston for thoughtful suggestions and comments on the paper and Drs Tom Benjamin and Dawei Li for the Sall2 antibody. Special thanks goes to
the faculty, technicians, residents and fellows of the division of Women's and Perinatal Pathology in the Department of Pathology at the Brigham and Women's Hospital, Boston, MA, for the
allocation of tissues. This work was supported by the National Cancer Institute [P50 CA105009, K08 CA108748 and R21 CA124688], Ovarian Cancer Research Fund (Individual Investigator Award and
Program Project Development Award), Phi Beta Psi Sorority Charitable Trust, Fannie E. Ripple Foundation, Robert and Deborah First Fund, Randi and Joel Cutler Ovarian Cancer Research Fund,
the Columbia Hospital for Women Research Foundation, Marsha Rivkin Foundation Scientific Scholar Award, AACR-George and Patricia Sehl Fellowship for Cancer Genetics Research and the American
Physicians Fellowship for Medicine in Israel- Claire and Emmanuel G Rosenblatt Foundation Grant.
Department of Medical Oncology, Center of Molecular Oncologic Pathology, Dana-Farber Cancer Institute, Boston, MA, USA
Department of Cancer Biology, Center of Proteomics, Dana-Farber Cancer Institute, Boston, MA, USA
Department of Pathology, Division of Women's and Perinatal Pathology, Brigham and Women's Hospital, Boston, MA, USA
M C Chang, M H Roh, D W Kindelberger, M S Hirsch, C P Crum & R Drapkin
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Anyone you share the following link with will be able to read this content:




