- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Nipah virus (NiV) is a highly pathogenic emergent paramyxovirus causing deadly encephalitis in humans. Its replication requires a constant supply of unassembled nucleoprotein (N0)
in complex with its viral chaperone, the phosphoprotein (P). To elucidate the chaperone function of P, we reconstituted NiV the N0–P core complex and determined its crystal structure. The
binding of the N-terminal region of P blocks the polymerization of N by interfering with subdomain exchange between N protomers and keeps N0 in an open conformation, ready to grasp an RNA
molecule. We found that a peptide derived from the N-binding region of P protects cells against viral infection and demonstrated by structure-based mutagenesis that this peptide acts by
inhibiting N0–P formation. These results provide new insights about the assembly of N along genomic RNA and validate the N0–P complex as a target for drug development. Access through your
institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print
issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to
local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT
BEING VIEWED BY OTHERS STRUCTURE OF THE NIPAH VIRUS POLYMERASE PHOSPHOPROTEIN COMPLEX Article Open access 07 October 2024 STRUCTURE OF THE NEWCASTLE DISEASE VIRUS L PROTEIN IN COMPLEX WITH
TETRAMERIC PHOSPHOPROTEIN Article Open access 10 March 2023 STRUCTURAL INSIGHT INTO MARBURG VIRUS NUCLEOPROTEIN–RNA COMPLEX FORMATION Article Open access 04 March 2022 ACCESSION CODES
PRIMARY ACCESSIONS PROTEIN DATA BANK * 4CO6 REFERENCED ACCESSIONS PROTEIN DATA BANK * 2WJ8 * 4BKK SWISS-PROT * A5H724 * B5AXP0 * D5FGX2 * F5BH21 * J7H328 * K9N0Q8 * O89339 * P04851 * P04865
* P17240 * P21737 * P27018 * Q03332 * Q08823 * Q66412 * Q77IS8 * Q88435 * Q99FY3 * Q9IK91 * Q9IK92 REFERENCES * Pringle, C.R. The order Mononegavirales: current status. _Arch. Virol._ 142,
2321–2326 (1997). CAS PubMed Google Scholar * Chua, K.B. et al. Nipah virus: a recently emergent deadly paramyxovirus. _Science_ 288, 1432–1435 (2000). Article CAS Google Scholar *
Morin, B., Rahmeh, A.A. & Whelan, S.P. Mechanism of RNA synthesis initiation by the vesicular stomatitis virus polymerase. _EMBO J._ 31, 1320–1329 (2012). Article CAS Google Scholar *
Arnheiter, H., Davis, N.L., Wertz, G., Schubert, M. & Lazzarini, R.A. Role of the nucleocapsid protein in regulating vesicular stomatitis virus RNA synthesis. _Cell_ 41, 259–267 (1985).
Article CAS Google Scholar * Tawar, R.G. et al. Crystal structure of a nucleocapsid-like nucleoprotein-RNA complex of respiratory syncytial virus. _Science_ 326, 1279–1283 (2009).
Article CAS Google Scholar * Albertini, A.A. et al. Crystal structure of the rabies virus nucleoprotein-RNA complex. _Science_ 313, 360–363 (2006). Article CAS Google Scholar * Green,
T.J., Zhang, X., Wertz, G.W. & Luo, M. Structure of the vesicular stomatitis virus nucleoprotein-RNA complex. _Science_ 313, 357–360 (2006). Article CAS Google Scholar * Desfosses,
A., Goret, G., Estrozi, L.F., Ruigrok, R.W. & Gutsche, I. Nucleoprotein-RNA orientation in the measles virus nucleocapsid by three-dimensional electron microscopy. _J. Virol._ 85,
1391–1395 (2011). Article CAS Google Scholar * Karlin, D., Ferron, F., Canard, B. & Longhi, S. Structural disorder and modular organization in _Paramyxovirinae_ N and P. _J. Gen.
Virol._ 84, 3239–3252 (2003). Article CAS Google Scholar * Jensen, M.R. et al. Intrinsic disorder in measles virus nucleocapsids. _Proc. Natl. Acad. Sci. USA_ 108, 9839–9844 (2011).
Article CAS Google Scholar * Communie, G. et al. Atomic resolution description of the interaction between the nucleoprotein and phosphoprotein of Hendra virus. _PLoS Pathog._ 9, e1003631
(2013). Article CAS Google Scholar * Curran, J., Marq, J.B. & Kolakofsky, D. An N-terminal domain of the Sendai paramyxovirus P protein acts as a chaperone for the NP protein during
the nascent chain assembly step of genome replication. _J. Virol._ 69, 849–855 (1995). CAS PubMed PubMed Central Google Scholar * Mavrakis, M. et al. Rabies virus chaperone:
identification of the phosphoprotein peptide that keeps nucleoprotein soluble and free from non-specific RNA. _Virology_ 349, 422–429 (2006). Article CAS Google Scholar * Gérard, F.C.A.
et al. Modular organization of rabies virus phosphoprotein. _J. Mol. Biol._ 388, 978–996 (2009). Article Google Scholar * Habchi, J., Mamelli, L., Darbon, H. & Longhi, S. Structural
disorder within Henipavirus nucleoprotein and phosphoprotein: from predictions to experimental assessment. _PLoS ONE_ 5, e11684 (2010). Article Google Scholar * Leyrat, C. et al. Ensemble
structure of the modular and flexible full-length vesicular stomatitis virus phosphoprotein. _J. Mol. Biol._ 423, 182–197 (2012). Article CAS Google Scholar * Leyrat, C. et al. Structure
of the vesicular stomatitis virus N-P complex. _PLoS Pathog._ 7, e1002248 (2011). Article CAS Google Scholar * Ruigrok, R.W., Crepin, T. & Kolakofsky, D. Nucleoproteins and
nucleocapsids of negative-strand RNA viruses. _Curr. Opin. Microbiol._ 14, 504–510 (2011). Article CAS Google Scholar * Rudolph, M.G. et al. Crystal structure of the Borna disease virus
nucleoprotein. _Structure_ 11, 1219–1226 (2003). Article CAS Google Scholar * Calain, P. & Roux, L. The rule of six, a basic feature for efficient replication of Sendai virus
defective interfering RNA. _J. Virol._ 67, 4822–4830 (1993). CAS PubMed PubMed Central Google Scholar * Halpin, K., Bankamp, B., Harcourt, B.H., Bellini, W.J. & Rota, P.A. Nipah
virus conforms to the rule of six in a minigenome replication assay. _J. Gen. Virol._ 85, 701–707 (2004). Article CAS Google Scholar * Lamb, R.A. in _Fields Virology_ 6th edn, Vol. 1
(eds. Knipe, D.M. & Howley, P.M.) 880–884 (Lippincott Williams & Wilkins, Philadelphia, 2013). * Karlin, D. & Belshaw, R. Detecting remote sequence homology in disordered
proteins: discovery of conserved motifs in the N-termini of _Mononegavirales_ phosphoproteins. _PLoS ONE_ 7, e31719 (2012). Article CAS Google Scholar * Katoh, K. & Standley, D.M.
MAFFT multiple sequence alignment software version 7: improvements in performance and usability. _Mol. Biol. Evol._ 30, 772–780 (2013). Article CAS Google Scholar * Marley, J., Lu, M.
& Bracken, C. A method for efficient isotopic labeling of recombinant proteins. _J. Biomol. NMR_ 20, 71–75 (2001). Article CAS Google Scholar * Wyatt, P.J. Submicrometer particle
sizing by multiangle light scattering following fractionation. _J. Colloid Interface Sci._ 197, 9–20 (1998). Article CAS Google Scholar * Uversky, V.N. Use of fast protein size-exclusion
liquid chromatography to study the unfolding of proteins which denature through the molten globule. _Biochemistry_ 32, 13288–13298 (1993). Article CAS Google Scholar * Konarev, P.,
Petoukhov, M., Volchkov, V. & Svergun, D.I. ATSAS 2.1, a program package for small-angle scattering data analysis. _J. Appl. Crystallogr._ 39, 277–286 (2006). Article CAS Google
Scholar * Svergun, D.I. Restoring low resolution structure of biological macromolecules from solution scattering using simulated annealing. _Biophys. J._ 76, 2879–2886 (1999). Article CAS
Google Scholar * Volkov, V.V. & Svergun, D.I. Uniqueness of _ab initio_ shape determination in small-angle scattering. _J. Appl. Crystallogr._ 36, 860–864 (2003). Article CAS Google
Scholar * Lescop, E., Schanda, P. & Brutscher, B. A set of BEST triple-resonance experiments for time-optimized protein resonance assignment. _J. Magn. Reson._ 187, 163–169 (2007).
Article CAS Google Scholar * Delaglio, F. et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. _J. Biomol. NMR_ 6, 277–293 (1995). Article CAS Google
Scholar * Jung, Y.S. & Zweckstetter, M. Mars: robust automatic backbone assignment of proteins. _J. Biomol. NMR_ 30, 11–23 (2004). Article CAS Google Scholar * Marsh, J.A., Singh,
V.K., Jia, Z. & Forman-Kay, J.D. Sensitivity of secondary structure propensities to sequence differences between α- and γ-synuclein: implications for fibrillation. _Protein Sci._ 15,
2795–2804 (2006). Article CAS Google Scholar * Jensen, M.R., Salmon, L., Nodet, G. & Blackledge, M. Defining conformational ensembles of intrinsically disordered and partially folded
proteins directly from chemical shifts. _J. Am. Chem. Soc._ 132, 1270–1272 (2010). Article CAS Google Scholar * Kabsch, W. Xds. _Acta Crystallogr. D Biol. Crystallogr._ 66, 125–132
(2010). Article CAS Google Scholar * Pape, T. & Schneider, T.R. HKL2MAP: a graphical user interface for phasing with SHELX programs. _J. Appl. Crystallogr._ 37, 843–844 (2004).
Article CAS Google Scholar * Terwilliger, T.C. et al. Iterative model building, structure refinement and density modification with the PHENIX AutoBuild wizard. _Acta Crystallogr. D Biol.
Crystallogr._ 64, 61–69 (2008). Article CAS Google Scholar * Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. _Acta Crystallogr. D
Biol. Crystallogr._ 66, 213–221 (2010). Article CAS Google Scholar * Afonine, P.V. et al. Towards automated crystallographic structure refinement with phenix.refine. _Acta Crystallogr. D
Biol. Crystallogr._ 68, 352–367 (2012). Article CAS Google Scholar * Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. _Acta Crystallogr. D Biol.
Crystallogr._ 60, 2126–2132 (2004). Article Google Scholar * Chen, V.B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. _Acta Crystallogr. D Biol.
Crystallogr._ 66, 12–21 (2010). Article CAS Google Scholar * Pettersen, E.F. et al. UCSF Chimera: a visualization system for exploratory research and analysis. _J. Comput. Chem._ 25,
1605–1612 (2004). Article CAS Google Scholar * Suhre, K. & Sanejouand, Y.H. ElNemo: a normal mode web server for protein movement analysis and the generation of templates for
molecular replacement. _Nucleic Acids Res._ 32, W610–W614 (2004). Article CAS Google Scholar * Abramoff, M.D., Magalhaes, P.J. & Ram, S.J. Image processing with ImageJ. _Biophoton.
Int._ 11, 36–42 (2004). Google Scholar Download references ACKNOWLEDGEMENTS We thank W. Burmeister and A. McCarthy for their help with X-ray data collection and C. Leyrat for discussions.
F.Y. was supported by a predoctoral fellowship from the Région Rhône-Alpes. This work was supported by grants from the French Agence Nationale de la Recherche to M.J. (ANR-07-001-01) and to
V.V. (ANR-09-MIEN-018-01), from the European Commission's FP7 program ANTIGONE (278976) to V.V. and from the Fondation Innovations en Infectiologie (FINOVI) to V.V. and M.J. This work
used the platforms of the Grenoble Instruct center (Integrated Structural Biology Grenoble; UMS3518 CNRS-CEA-UJF-EMBL) with support from The French Infrastructure for Integrated Structural
Biology (FRISBI) (ANR-10-INSB-05-02) and The Alliance Grenobloise pour la Biologie Structurale et Cellulaire Intégrées (GRAL) (ANR-10-LABX-49-01) within the Grenoble Partnership for
Structural Biology (PSB). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Université Grenoble Alpes, Unit of Virus Host Cell Interactions, Grenoble, France Filip Yabukarski, Nicolas
Tarbouriech, Jean-Marie Bourhis, Rob W H Ruigrok & Marc Jamin * CNRS, Unit of Virus Host Cell Interactions, Grenoble, France Filip Yabukarski, Nicolas Tarbouriech, Jean-Marie Bourhis,
Rob W H Ruigrok & Marc Jamin * International Centre for Research in Infectiology (CIRI), INSERM U1111–CNRS UMR5308, Université Lyon 1, Ecole Normale Supérieure de Lyon, Lyon, France
Philip Lawrence & Viktor Volchkov * Université Grenoble Alpes, Institut de Biologie Structurale, Grenoble, France Elise Delaforge, Malene Ringkjøbing Jensen & Martin Blackledge *
CNRS, Institut de Biologie Structurale, Grenoble, France Elise Delaforge, Malene Ringkjøbing Jensen & Martin Blackledge * Commissariat à l′Énergie Atomique (CEA), Institut de Biologie
Structurale, Grenoble, France Elise Delaforge, Malene Ringkjøbing Jensen & Martin Blackledge Authors * Filip Yabukarski View author publications You can also search for this author
inPubMed Google Scholar * Philip Lawrence View author publications You can also search for this author inPubMed Google Scholar * Nicolas Tarbouriech View author publications You can also
search for this author inPubMed Google Scholar * Jean-Marie Bourhis View author publications You can also search for this author inPubMed Google Scholar * Elise Delaforge View author
publications You can also search for this author inPubMed Google Scholar * Malene Ringkjøbing Jensen View author publications You can also search for this author inPubMed Google Scholar *
Rob W H Ruigrok View author publications You can also search for this author inPubMed Google Scholar * Martin Blackledge View author publications You can also search for this author inPubMed
Google Scholar * Viktor Volchkov View author publications You can also search for this author inPubMed Google Scholar * Marc Jamin View author publications You can also search for this
author inPubMed Google Scholar CONTRIBUTIONS F.Y., P.L., M.R.J., R.W.H.R., M.B., V.V. and M.J. designed all experiments. F.Y., P.L., E.D. and M.R.J. performed the experiments. P.L. performed
BSL-4 experiments. F.Y., P.L., N.T., J.-M.B., M.R.J., R.W.H.R., M.B., V.V. and M.J. contributed to data analysis. F.Y., P.L., M.R.J., M.B., V.V. and M.J. wrote the paper. CORRESPONDING
AUTHORS Correspondence to Viktor Volchkov or Marc Jamin. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. INTEGRATED SUPPLEMENTARY INFORMATION
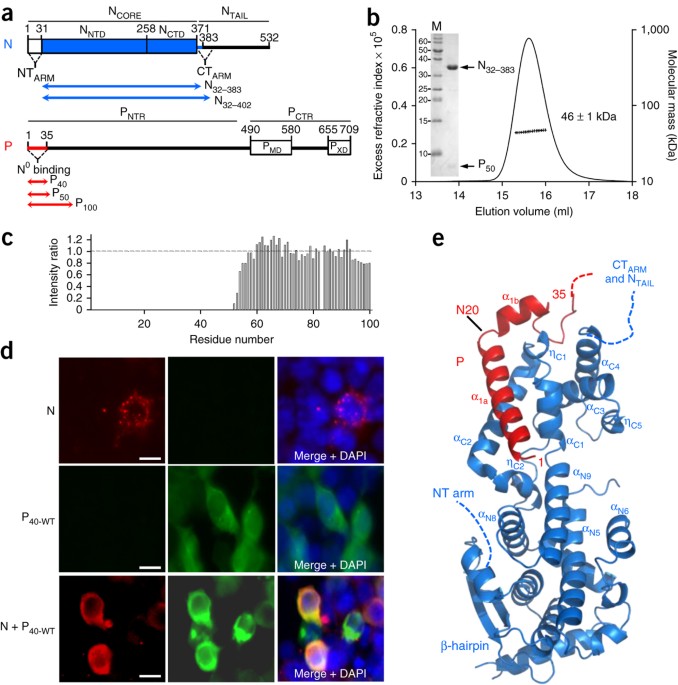
SUPPLEMENTARY FIGURE 1 STRUCTURAL CHARACTERIZATION OF NIV N0–P IN SOLUTION AND IN CRYSTAL. (A) SAXS analysis of the N32-3830-P50 complex. The Guinier plot for complex concentrations of 0.55,
1.1, 1.6 and 2.4 mg.mL-1 shows no evidence for aggregation or intermolecular interactions. The averaged intensity at zero angle corrected for protein concentration (I(0)/C) value of 46 ± 1
kDa is in good agreement with the theoretical molecular mass of the heterodimeric complex (45,613 Da). (B) Superposition of the experimental SAXS curve in black (2.4 mg.mL-1) with the
theoretical curve (in red) back calculated for the averaged _ab initio_ bead model (20 independent models; normalized spatial discrepancy (NSD) < 1.0) generated with DAMMIN28 and
DAMAVER29. (C) The heterodimeric N32-3830-P50 complex has a bean shape in solution. The _ab initio_ bead model generated from SAXS data (in grey) accommodates a single N32-3830-P50 copy from
the crystal. (D) Comparison of the 2D 1H-15N heteronuclear single-quantum coherence (HSQC) NMR spectra of free P100 (in red) and of P100 bound to N32-402(in blue). (E) Electrostatic surface
potential of NiV and RSV N (PDB code 2WJ8; ref. 5) proteins at ± 5 kTe-1: blue (basic), white (neutral) and red (acidic). The hypothetical RNA binding site is indicated in NiV N, and bound
RNA in the RSV complex is shown in orange. (F) Western blot using in-house henipavirus specific rabbit anti-N antibody with cell lysates from Nipah infected Vero E6 cells lysed 48h
post-infection (NiV, left lane) compared to uninfected cells (NI, right lane). Theoretical molecular mass of the NiV N protein is 58,168 Da. SUPPLEMENTARY FIGURE 2 NIV P100 IS GLOBALLY
DISORDERED IN SOLUTION BUT CONTAINS FLUCTUATING Α-HELICAL ELEMENTS. (A,B). Molecular size. The hydrodynamic radius of 2.6 ± 0.5 nm measured by SEC (A) and the radius of gyration of 3.1 ± 0.5
nm measured by SAXS (B) are larger than expected for a compact domain of this size. SAXS profiles were recorded at 0.3, 0.5 and 0.6 mg.mL-1. (C) NMR spectroscopy. The poor chemical shift
dispersion of amide 1H resonances in the heteronuclear single quantum coherence (HSQC) NMR spectrum (Supplementary Fig. 1d) is typical of disordered proteins. The secondary structure
propensity (SSP) parameter calculated from Cα and Cβ secondary chemical shifts indicates the presence of fluctuating helices (red boxes). The position of helices in the N-terminal region of
P in the crystal structure of the N32-3830-P50 complex is shown below. (D) The N0-binding region of _Paramyxovirinae_ P contains two conserved motifs (Soyuz1 and Soyuz2)23. Conserved
residues are colored according to their properties: acidic residues in violet, basic in red, hydrophobic in blue, polar in green and glycine in orange. G10 and I17 (larger letters) were
mutated into arginine in order to destabilize the N0-P complex. SUPPLEMENTARY FIGURE 3 THE CRYSTALLOGRAPHIC ASYMMETRIC UNIT CONTAINS THREE COPIES OF THE N32–3830–P50 COMPLEX. (A) Initial
plate cluster used for microseeding. (B) Typical crystal obtained for the N32-3830-P50 from microseeding and used for data collection. (C) Overall view of crystal packing of the N32-3830-P50
complex. The three copies of the complex in the asymmetric unit are colored in blue and red. The packing shows no indication of specific oligomerization of N in the crystal. (D,E).
Experimental selenium SAD phased density map of P50 (D) and N0 (E) in the N32-3830-P50 complex. The final model after refinement is superposed on the map at contour level 1 σ. Only one N0-P
copy from the asymmetric unit is represented. (F,G) Final 2Fo-Fc difference electron density map of P50 (F) and N0 (G) in the N32-3830-P50 complex contoured at 1 σ. (H). Comparison of the
different copies of the N32-3830-P50 complex present in the asymmetric unit. Two copies are well defined in the SAD phased map, whereas NNTD of the third one is less densely packed in the
crystal and the corresponding electron density is ill-defined. (I) Structural overlay of the three copies of N. Slightly different orientations of secondary structure elements in NNTD
suggest that this region of the protein is dynamic, at least in the absence of RNA. SUPPLEMENTARY FIGURE 4 COMPARISON OF NNVS N 3D FOLD. (A) Conserved architecture of the N proteins: NiV,
Nipah virus (_Paramyxoviridae_ - _Paramyxovirinae_); RSV, respiratory syncytial virus (_Paramyxoviridae_ - _Pneumovirinae_) (PDB code 2WJ8; ref 5); BDV, Borna disease virus (_Bornaviridae_)
(PDB code 1N93; ref 19); VSV, vesicular stomatitis virus (_Rhabdoviridae_) (PDB code 2GIC; ref 7). Pairwise structural alignments led us to define four subdomains in the N core, namely
NNTD1, NNTD2, NNTD3 and NCTD. The structures are similarly oriented and colored according to subdomains. VSV N contains an additional C-terminal subdomain (NCTD2 in purple) comprising three
α-helices, which form the surface of binding of the C-terminal domain of P. (B-D). Superpositions of subdomains from the different proteins. The NiV N subdomains are shown in the same colors
as in panel a and the corresponding subdomains from the other viruses are shown in grey: NNTD1 (B), NNTD3 (C) and NCTD (D). NNV N proteins differ mainly in the relative orientations of
these three domains and the structure of the variable NNTD2 region. (E) Pairwise comparison of NiV N and human RSV N. Structure-based sequence alignment of NiV N (PDB code 4CO6) and RSV N
(PDB code 2WJ8; ref 5). Secondary structure elements and residue numbering of NiV N are indicated above and secondary structure elements of RSV are indicated below. SUPPLEMENTARY FIGURE 5
RNA GRASP AND HINGE MOTIONS. (A) Normal mode analysis (NMA) and hypothetical hinge motion in NiV N. Elastic network NMA was carried out on the N0 molecule to explore its motions45.
Representative models of the two lowest-frequency modes show motions of NNTD relative to NCTD with a hinge at the junction between the two domains (red circle) in agreement with a mechanism
of closure of the RNA binding cavity. (B) The second lowest mode (mode 8) captures a rotation of NNTD, which agrees with the hypothetical closure of the protein around the RNA. The initial
model (in grey) superposes with the crystal structure of NiV N0 in blue. (C) After displacement along mode 8, the model (in wheat) superposes with RSV N structure taken from the N-RNA
complex (in light blue). (D) Close-up of RSV N-RNA complex showing that helices αN6a and αN9 pack against the RNA molecule. G241 and G245 are shown in yellow. (E) Close-up of NiV N in the
N0-P crystal structure (left panel) and in a hypothetical closed conformation (right panel). The RNA molecule (in grey) is positioned against NCTD as in RSV NC. Several residues of conserved
motif 3 and conserved D254 interfere with base 1 binding in the closed form. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TEXT AND FIGURES Supplementary Figures 1–5 and Supplementary Tables 1
and 2 (PDF 4733 kb) PROPOSED MECHANISM OF CONFORMATIONAL CHANGE IN NIV N UPON RNA BINDING. The animation shows the proposed hinge motion of N lobes upon RNA binding with a pivot point at the
junction of NNTD and NCTD. The RNA molecule is positioned against NCTD as in RSV N-RNA complex, but only 6 nt are shown in accordance with the rule of six enforced in the subfamily
_Paramyxovirinae_. (WMV 48531 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yabukarski, F., Lawrence, P., Tarbouriech, N. _et al._ Structure of
Nipah virus unassembled nucleoprotein in complex with its viral chaperone. _Nat Struct Mol Biol_ 21, 754–759 (2014). https://doi.org/10.1038/nsmb.2868 Download citation * Received: 10 May
2014 * Accepted: 03 July 2014 * Published: 10 August 2014 * Issue Date: September 2014 * DOI: https://doi.org/10.1038/nsmb.2868 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative



