- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
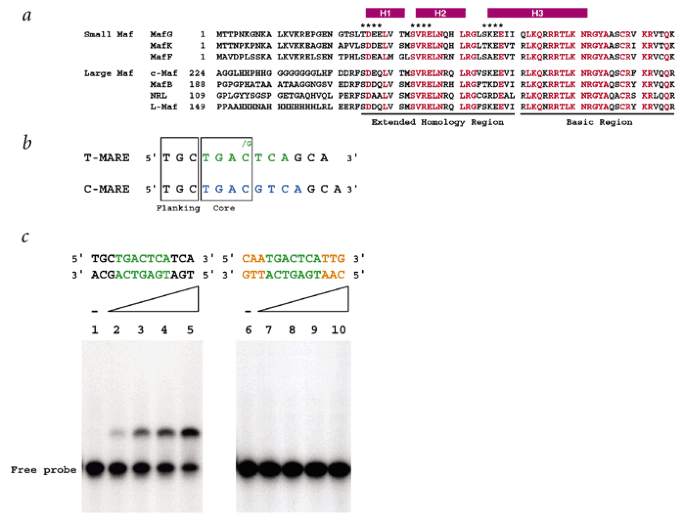
ABSTRACT The Maf family proteins, which constitute a subgroup of basic region-leucine zipper (bZIP) proteins, function as transcriptional regulators of cellular differentiation. Together
with the basic region, the Maf extended homology region (EHR), conserved only within the Maf family, defines the DNA binding specific to Mafs. Here we present the first NMR-derived structure
of the DNA-binding domain (residues 1–76) of MafG, which contains the EHR and the basic region. The structure consists of three α-helices and resembles the fold of the DNA-binding domain of
Skn-1, a developmental transcription factor of _Caenorhabditis elegans_. The structural similarity between MafG and Skn-1 enables us to propose a possible mechanism by which Maf family
proteins recognize their consensus DNA sequences. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS
Access through your institution Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on
SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about
institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS STRUCTURE OF THE HUMAN SAGA COACTIVATOR COMPLEX Article Open access 22 November
2021 COMPARATIVE STRUCTURAL INSIGHTS AND FUNCTIONAL ANALYSIS FOR THE DISTINCT UNBOUND STATES OF HUMAN AGO PROTEINS Article Open access 19 March 2025 STRUCTURE AND ASSEMBLY OF THE NOT10:11
MODULE OF THE CCR4-NOT COMPLEX Article Open access 17 July 2023 ACCESSION CODES ACCESSIONS GENBANK/EMBL/DDBJ * O42290 * O54790 * O54791 * P34707 * P54841 * P54846 * Q61827 * Q92171 PROTEIN
DATA BANK * 1K1V * 1SKN REFERENCES * Motohashi, H., Shavit, J.A., Igarashi, K., Yamamoto, M. & Engel, J.D. _NucleicAcids Res._ 25, 2953–2959 (1997). Article CAS Google Scholar *
Blank, V. & Andrews, N.C. _Trends Biochem. Sci._ 22, 437–441 (1997). Article CAS Google Scholar * Motohashi, H., Katsuoka, F., Shavit, J.A., Engel, J.D. & Yamamoto, M. _Cell_ 103,
865–875 (2000). Article CAS Google Scholar * Kataoka, K. et al. _Mol. Cell. Biol._ 15, 2180–2190 (1995). Article CAS Google Scholar * Kataoka, K., Fujiwara, K.T., Noda, M. &
Nishizawa, M. _Mol. Cell. Biol._ 14,7581–7591 (1994). Article CAS Google Scholar * Kerppola, T.K. & Curran, T. _Oncogene_ 9, 3149–3158 (1994). CAS PubMed Google Scholar * Wishart,
D.S. & Sykes, B.D. _Methods Enzymol._ 239, 36–81 (1994). Google Scholar * Farrow, N.A. et al. _Biochemistry_ 33, 5984–6003 (1994). Article CAS Google Scholar * Harper, E.T. &
Rose, G.D. _Biochemistry_ 32, 7605–7609 (1993). Article CAS Google Scholar * Wintjens, R. & Rooman, M. _J. Mol. Biol._ 262, 294–313 (1996). Article CAS Google Scholar * Lo, M.C.,
Ha, S., Pelczer, I., Pal, S. & Walker, S. _Proc. Natl. Acad. Sci. USA_ 95, 8455–8460 (1998). Article CAS Google Scholar * Rupert, P.B., Daughdrill, G.W., Bowerman, B. & Matthews,
B.W. _Nature Struct. Biol._ 5, 484–491 (1998). Article CAS Google Scholar * Ellenberger, T.E., Brandl, C.J., Struhl, K. & Harrison, S.C. _Cell_ 71, 1223–1237 (1992). Article CAS
Google Scholar * Glover, J.N. & Harrison, S.C. _Nature_ 373, 257–261 (1995). Article CAS Google Scholar * Delaglio, F. et al. _J. Biomol. NMR_ 6, 277–293 (1995). Article CAS Google
Scholar * Garrett, D.S., Powers, R., Gronenborn, A.M. & Clore, G.M. _J. Magn. Reson._ 95,214–220 (1991). CAS Google Scholar * Sattler, M., Schleucher, J. & Griesinger, C. _Prog.
NMR Spect._ 34, 93–158 (1999). Article CAS Google Scholar * Neri, D., Szyperski, T., Otting, G., Senn, H. & Wüthrich, K. _Biochemistry_ 28,7510–7516 (1989). Article CAS Google
Scholar * Tomomori, C. et al. _Nature Struct. Biol._ 6, 729–734 (1999). Article CAS Google Scholar * Vuister, G.W. & Bax, A. _J. Am. Chem. Soc._ 115, 7772–7777 (1993). Article CAS
Google Scholar * Nilges, M., Clore, G.M. & Gronenborn, A.M. _FEBS Lett._ 229, 317–324 (1988). Article CAS Google Scholar * Brünger, A.T. X-PLOR version 3.1: A system for X-ray
crystallography and NMR (Yale University Press, New Haven; 1993). * Koradi, R., Billeter, M. & Wüthrich, K. _J. Mol. Graph._ 14, 51–55 (1996). Article CAS Google Scholar * Kraulis,
P.J. _J. Appl. Crystallogr._ 24, 946–950 (1991). Article Google Scholar * Merritt, E.A. & Bacon, D.J. _Methods Enzymol._ 277, 505–524 (1997). Article CAS Google Scholar * Nicholls,
A., Sharp, K.A. & Honig, B. _Proteins_ 11, 281–296 (1991). Article CAS Google Scholar * Azam, T.A. & Ishihama, A. _J. Biol. Chem._ 274, 33105–33113 (1999). Article CAS Google
Scholar * Johnson, M.L., Correia, J.J., Yphantis, D.A. & Halvorson, H.R. _Biophys. J._ 36, 575–588 (1981). Article CAS Google Scholar * Laue, T.M., Bhairavi, D.S., Ridgeway, T.M.
& Pelletier, S.L. In _Analytical ultracentrifugation in biochemistry and polymer science_ (eds Harding, S.E., Rowe, A.J. & Horton, J.C) 90–125 (Royal Society of Chemistry, London;
1992). Google Scholar * Brooks, B.R. et al. _J. Comp. Chem._ 4, 187–217 (1983). Article CAS Google Scholar * Laskowski, R.A., Rullmann, J.A.C., MacArthur, M.W., Kaptein, R. &
Thornton,J.M. _J. Biomol. NMR_ 8, 477–486 (1996). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank E. Arai and F. Arisaka for ultracentrifuge analysis, T. Maeda
for useful discussion, K. Yap for providing a program to calculate interhelical angles and T. O'Connor for critical reading of the manuscript. This work was supported by grants from
JSPS and TARA (T.T.); the Ministry of Education, Science, Sports and Culture of Japan (H.M. and M.Y.); JSPS and CREST (M.Y.); and PROBRAIN (H.M.). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS
* Institute of Basic Medical Science, University of Tsukuba, Tsukuba, 305-8575, Ibaraki, Japan Hideki Kusunoki, Hozumi Motohashi, Fumiki Katsuoka & Masayuki Yamamoto * Center for
Tsukuba Advanced Research Alliance, University of Tsukuba, Tsukuba, 305-8577, Ibaraki, Japan Hideki Kusunoki, Hozumi Motohashi, Masayuki Yamamoto & Toshiyuki Tanaka * Institute of
Applied Biochemistry, University of Tsukuba, Tsukuba, 305-8572, Ibaraki, Japan Akio Morohashi & Toshiyuki Tanaka * Banyu Tsukuba Research Institute, Tsukuba, 300-2611, Ibaraki, Japan
Akio Morohashi Authors * Hideki Kusunoki View author publications You can also search for this author inPubMed Google Scholar * Hozumi Motohashi View author publications You can also search
for this author inPubMed Google Scholar * Fumiki Katsuoka View author publications You can also search for this author inPubMed Google Scholar * Akio Morohashi View author publications You
can also search for this author inPubMed Google Scholar * Masayuki Yamamoto View author publications You can also search for this author inPubMed Google Scholar * Toshiyuki Tanaka View
author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Toshiyuki Tanaka. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare no competing financial interests. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kusunoki, H., Motohashi, H., Katsuoka, F. _et al._ Solution
structure of the DNA-binding domain of MafG. _Nat Struct Mol Biol_ 9, 252–256 (2002). https://doi.org/10.1038/nsb771 Download citation * Received: 02 October 2001 * Accepted: 24 January 2002
* Published: 04 March 2002 * Issue Date: 01 April 2002 * DOI: https://doi.org/10.1038/nsb771 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content:
Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative







