- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Many clinical response measures are used in clinical trials: relapse-derived measures reflect the clinical effect of inflammatory activity, whereas disability-derived measures reflect the
effect of neurodegeneration
Among the neuroimaging measures used in clinical trials, lesion-derived metrics capture inflammatory activity, whereas brain atrophy measures reflect neurodegeneration; the choice of measure
should reflect the drug's mechanism of action
Owing to technical, financial and logistical barriers, most of the clinical and neuroimaging response measures used in trials are not used in the clinical setting
All clinical and neuroimaging response measures used in the clinic should have a clear meaning at the individual level
Combined (clinical and MRI) outcomes can be used in both trial and clinic settings, although their increased sensitivity to detect treatment effects particularly favours their use in trials
The use of patient-reported outcome measures is important because they capture the impact (and effects) of the intervention on clinical disability, MRI parameters, daily activities and
quality of life
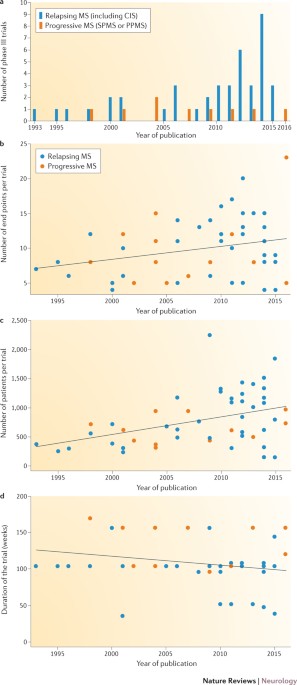
Increasing numbers of drugs are being developed for the treatment of multiple sclerosis (MS). Measurement of relevant outcomes is key for assessing the efficacy of new drugs in clinical
trials and for monitoring responses to disease-modifying drugs in individual patients. Most outcomes used in trial and clinical settings reflect either clinical or neuroimaging aspects of MS
(such as relapse and accrual of disability or the presence of visible inflammation and brain tissue loss, respectively). However, most measures employed in clinical trials to assess
treatment effects are not used in routine practice. In clinical trials, the appropriate choice of outcome measures is crucial because the results determine whether a drug is considered
effective and therefore worthy of further development; in the clinic, outcome measures can guide treatment decisions, such as choosing a first-line disease-modifying drug or escalating to
second-line treatment. This Review discusses clinical, neuroimaging and composite outcome measures for MS, including patient-reported outcome measures, used in both trials and the clinical
setting. Its aim is to help clinicians and researchers navigate through the multiple options encountered when choosing an outcome measure. Barriers and limitations that need to be overcome
to translate trial outcome measures into the clinical setting are also discussed.
C.T. acknowledges financial support for her postdoctoral research fellowship from the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). J.C., F.B., A.J.T. and
O.C. acknowledge funding from the UK National Institute for Health Research (NIHR) University College London Hospitals (UCLH) Biomedical Research Centre (BRC). J.C. also acknowledges support
from the NIHR Efficacy and Mechanism Evaluation and Health Technology Assessment Programmes and the UK and US National Multiple Sclerosis (MS) Societies. F.B. also acknowledges funding from
the European Community Horizon 2020 (European Union Framework Programme for Research and Innovation), the Innovative Medicines Initiative (IMI) Horizon 2020 programme, amyloid imaging to
prevent Alzheimer's disease (AMYPAD), and the Dutch MS Society. A.J.T. and O.C. acknowledge funding from the UK MS Society.
Queen Square Multiple Sclerosis Centre, University College of London Institute of Neurology, London, WC1B 5EH, UK
Carmen Tur, Marcello Moccia, Frederik Barkhof, Jeremy Chataway & Olga Ciccarelli
Department of Neuroscience, Multiple Sclerosis Clinical Care and Research Centre, Federico II University, Via Sergio Pansini 5, Naples, 80131, Italy
Institute of Healthcare Engineering, University College London, Engineering Front Building, Room 2.01, 2nd Floor, Torrington Place, London, WC1E 7JE, UK
Vrije Universiteit (VU) University Medical Centre — Radiology and Nuclear Medicine, Van der Boechorststraat 7 F/A-114, Amsterdam, 1081 BT, Netherlands
National Institute for Health Research, University College London Hospitals Biomedical Research Centre, 170 Tottenham Court Rd, London, W1T 7HA, UK
Frederik Barkhof, Jeremy Chataway, Alan J. Thompson & Olga Ciccarelli
Department of Neurology and Neuroimmunology, Multiple Sclerosis Centre of Catalonia, Vall d'Hebron University Hospital, 119–129, Barcelona, 08035, Spain
Department of Brain Repair and Rehabilitation, University College London Faculty of Brain Sciences, Institute of Neurology, Queen Square, London, WC1N 3BG, UK
C.T., M.M. and O.C. researched data for the article. All authors wrote the manuscript and contributed substantially to discussions of content and review or editing of the manuscript before
submission.
C.T. declares that she has received honoraria and support for travelling from Bayer Schering, Biogen, Ismar Healthcare, Merck Serono, Novartis, Sanofi–Aventis, Serono Foundation and Teva.
M.M. declares that he has received honoraria and support for travelling from Almirall, Coloplast, Genzyme and Merck Serono. F.B. declares that he has received consulting fees for
participating in steering committees, data-safety monitoring boards and advisory boards from Bayer Schering, Biogen, Genzyme, Jansen Research, Merck Serono, Novartis, Roche, Sanofi–Aventis,
Synthon and Teva. J.C. declares that he has been a principal investigator for trials in multiple sclerosis funded by Biogen, Novartis and Receptos and additionally received an investigator
grant from Novartis; he has also participated in advisory boards and/or received consultancy fees from Apitope, Biogen, MedDay, Merck and Roche. J.S.-G. declares that he has received
speaker's honoraria from and/or participated in advisory boards for Almirall, Biogen, Celgene, Genzyme, Merck, Novartis and Teva. A.J.T. declares that he has received honoraria and/or
support for travel for consultancy from Biogen (Optum Insight), Eisai and Excemed and support for travel from the International Progressive Multiple Sclerosis Alliance (as chair of their
Scientific Steering Committee) and from the US National Multiple Sclerosis Society (as a member of their Research Programs Advisory Committee); he also receives an honorarium from SAGE
Publishers as Editor in Chief of Multiple Sclerosis Journal. O.C. declares that she has received fees for consultancy from Biogen, Genzyme, Novartis, Roche and Teva and an honorarium for her
position as an associate editor of Neurology.
Clinical outcome measures in phase III trials in relapsing-remitting (RR) MS (DOC 371 kb)
Clinical outcome measures in phase III trials in clinically isolated syndromes (CIS) (DOC 110 kb)
Clinical outcome measures in phase III trials in progressive MS (DOC 220 kb)
Brain MRI outcome measures in phase III trials in relapsing-remitting MS (DOC 318 kb)
Brain MRI outcome measures in phase III trials in CIS (DOC 104 kb)
Brain MRI outcome measures in phase III trials in progressive MS (DOC 121 kb)
Phase II and 3 trials that used spinal cord MRI outcomes* (DOC 68 kb)
Past and ongoing phase II and III trials which use Optical Coherence Tomography (OCT)-related measures. (DOC 90 kb)
Anyone you share the following link with will be able to read this content:






