- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Access through your institution Buy or subscribe Frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS) are thought to belong to a spectrum of neurodegenerative disorders with
shared clinicopathological and genetic features. Recent studies have identified a class of disorders collectively termed c9FTD/ALS, which are caused by GGGGCC hexanucleotide repeat
expansions in the _C9orf72_ gene. Research groups led by Leonard Petrucelli of the Mayo Clinic and Dieter Edbauer of Ludwig–Maximilians University have now independently shown that
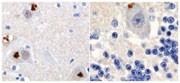
translation of the GGGGCC expansions in _C9orf72_ results in the characteristic TDP-43-negative neuronal inclusions of c9FTD/ALS (the protein composition of which was previously unknown),
and may be an underlying pathogenetic mechanism in these diseases. “Targeting RAN-translated peptides, their aggregation or the RNA structures necessary for their formation may show great
promise as treatment strategies in patients with c9FTD/ALS,” comments Petrucelli, noting that anti-C9RANT-immunoreactive aggregates are specific to this disease. “Therapies to target C9RANT
aggregates may [also] prove beneficial to _C9orf72_ mutation carriers,” he continues. “Detection of C9RANT in cerebrospinal fluid may provide a valuable diagnostic and prognostic tool for
identifying patients carrying the _C9orf72_ repeat expansion and then tracking the progression of the disease in at-risk individuals.” Petrucelli also highlights the key role of immunoassays
for detection of C9RANT in cerebrospinal fluid. His team plans to conduct further evaluations of their preliminary findings to confirm whether C9RANT peptides are neurotoxic. This is a
preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $209.00 per
year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated
during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support ORIGINAL RESEARCH PAPERS * Ash, P. E. _ et al_.
Unconventional translation of _C9ORF72_ GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. _Neuron_ doi:10.1016/j.neuron.2013.02.004 * Mori, K. _ et al_. The _C9orf72_
GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. _Science_ doi:10.1126/science.1232927 Download references Authors * Ellen Bible View author publications
You can also search for this author inPubMed Google Scholar RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Bible, E. Researchers identify the protein in
c9FTD/ALS inclusions. _Nat Rev Neurol_ 9, 183 (2013). https://doi.org/10.1038/nrneurol.2013.39 Download citation * Published: 12 March 2013 * Issue Date: April 2013 * DOI:
https://doi.org/10.1038/nrneurol.2013.39 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative