- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
KEY POINTS * Down syndrome results from the presence of an extra copy or major portion of human chromosome 21 (_Homo sapiens_ autosome 21 (HSA21)), producing a genetic imbalance. * Our
understanding of Down syndrome has shifted from a causative gene-based view to one in which genes, deregulation of non-coding elements and epigenetic factors influence the disease phenotype.
* In Down syndrome, the ability to keep incoming information online, the performance of mental computations on such information and the storage of this information for future use are
disrupted. * The size of certain brain regions affected in Down syndrome is correlated with performance in tests of intelligence and language. * HSA21-encoded proteins with master regulator
functions, such as transcription or splicing efficiency of specific mRNA, may exert a combinatorial effect by promoting or inhibiting the transcription or splicing of their targets, thus
spreading the effect of trisomy 21 to genes outside HSA21. * Many strategies have been used to model Down syndrome in mice. Mouse trisomies allow analysis of Down syndrome neurobiology, the
importance of specific chromosomal regions and understanding the efficacy of treatments. Single-gene transgenesis is a complementary approach in which we may better dissect the gene-specific
effects of recapitulated Down syndrome phenotypes. * In the past few years, we have made notable advances in finding a 'cure' for Down syndrome-linked intellectual disability
based on symptomatic alleviation and individual gene function rescue. ABSTRACT Down syndrome is the most common form of intellectual disability and results from one of the most complex
genetic perturbations that is compatible with survival, trisomy 21. The study of brain dysfunction in this disorder has largely been based on a gene discovery approach, but we are now moving
into an era of functional genome exploration, in which the effects of individual genes are being studied alongside the effects of deregulated non-coding genetic elements and epigenetic
influences. Also, new data from functional neuroimaging studies are challenging our views of the cognitive phenotypes associated with Down syndrome and their pathophysiological correlates.
These advances hold promise for the development of treatments for intellectual disability. Access through your institution Buy or subscribe This is a preview of subscription content, access
via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $189.00 per year only $15.75 per issue Learn more Buy
this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: *
Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS CONSEQUENCES OF TRISOMY 21 FOR BRAIN DEVELOPMENT IN DOWN
SYNDROME Article 08 October 2024 THE CONTRIBUTION OF COPY NUMBER VARIANTS TO PSYCHIATRIC SYMPTOMS AND COGNITIVE ABILITY Article 03 February 2023 _ELP2_ MUTATIONS PERTURB THE EPITRANSCRIPTOME
AND LEAD TO A COMPLEX NEURODEVELOPMENTAL PHENOTYPE Article Open access 11 May 2021 REFERENCES * Megarbane, A. et al. The 50th anniversary of the discovery of trisomy 21: the past, present,
and future of research and treatment of Down syndrome. _Genet. Med._ 11, 611–616 (2009). Article PubMed Google Scholar * Lott, I. T. & Dierssen, M. Cognitive deficits and associated
neurological complications in individuals with Down's syndrome. _Lancet Neurol._ 9, 623–633 (2010). Article PubMed Google Scholar * Khoshnood, B., Greenlees, R., Loane, M. &
Dolk, H. Paper 2: EUROCAT public health indicators for congenital anomalies in Europe. _Birth Defects Res. A Clin. Mol. Teratol._ 91, S16–S22 (2011). Article CAS PubMed PubMed Central
Google Scholar * Parker, S. E. et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. _Birth Defects Res. A Clin. Mol. Teratol._ 88,
1008–1016 (2010). Article CAS PubMed Google Scholar * Hattori, M. et al. The DNA sequence of human chromosome 21. _Nature_ 405, 311–319 (2000). A STUDY THAT REVEALED THE SEQUENCE AND
GENE CATALOGUE OF THE LONG ARM OF CHROMOSOME 21, WHICH WERE CRUCIAL FINDINGS FOR DOWN SYNDROME RESEARCH. Article CAS PubMed Google Scholar * Lott, I. T. Neurological phenotypes for Down
syndrome across the life span. _Prog. Brain Res._ 197, 101–121 (2012). Article CAS PubMed PubMed Central Google Scholar * Vicari, S., Bellucci, S. & Carlesimo, G. A. Visual and
spatial long-term memory: differential pattern of impairments in Williams and Down syndromes. _Dev. Med. Child Neurol._ 47, 305–311 (2005). A PAPER DETAILING THE DIFFERENCES IN THE COGNITIVE
PROFILES IN DOWN AND WILLIAMS SYNDROMES, WHICH HAVE HELPED TO GAIN INSIGHT INTO THE HIPPOCAMPAL FUNCTION AND THE STRUCTURE–FUNCTION RELATIONSHIP IN INTELLECTUAL DISABILITY. Article PubMed
Google Scholar * Conners, F. A., Moore, M. S., Loveall, S. J. & Merrill, E. C. Memory profiles of Down, Williams, and fragile X syndromes: implications for reading development. _J.
Dev. Behav. Pediatr._ 32, 405–417 (2011). Article PubMed Google Scholar * Edgin, J. O., Pennington, B. F. & Mervis, C. B. Neuropsychological components of intellectual disability: the
contributions of immediate, working, and associative memory. _J. Intellect. Disabil. Res._ 54, 406–417 (2010). Article PubMed PubMed Central Google Scholar * Baddeley, A. & Jarrold,
C. Working memory and Down syndrome. _J. Intellect. Disabil. Res._ 51, 925–931 (2007). BADDELEY PROPOSED THAT WORKING MEMORY COULD BE DIVIDED INTO THREE SUBSYSTEMS: ONE THAT IS CONCERNED
WITH VERBAL AND ACOUSTIC INFORMATION (THE PHONOLOGICAL LOOP); A SECOND THAT IS CONCERNED WITH THE VISUOSPATIAL SKETCHPAD; AND A THIRD THAT IS AN ATTENTIONALLY LIMITED CONTROL SYSTEM (THE
CENTRAL EXECUTIVE), ON WHICH THE FIRST AND SECOND ARE DEPENDENT. A FOURTH SUBSYSTEM, THE EPISODIC BUFFER, HAS NOW BEEN ADDED TO THIS OPERATIONAL MODEL. Article CAS PubMed Google Scholar
* Lanfranchi, S., Baddeley, A., Gathercole, S. & Vianello, R. Working memory in Down syndrome: is there a dual task deficit? _J. Intellect. Disabil. Res._ 56, 157–166 (2011). Article
PubMed Google Scholar * Dierssen, M., Herault, Y. & Estivill, X. Aneuploidy: from a physiological mechanism of variance to Down syndrome. _Physiol. Rev._ 89, 887–920 (2009). Article
CAS PubMed Google Scholar * Chapman, R. S. & Hesketh, L. J. Behavioral phenotype of individuals with Down syndrome. _Ment. Retard. Dev. Disabil. Res. Rev._ 6, 84–95 (2000). Article
CAS PubMed Google Scholar * Laws, G. & Bishop, D. V. A comparison of language abilities in adolescents with Down syndrome and children with specific language impairment. _J. Speech
Lang. Hear. Res._ 46, 1324–1339 (2003). Article PubMed Google Scholar * Purser, H. R. M. & Jarrold, C. Impaired verbal short-term memory in Down syndrome reflects a capacity
limitation rather than atypically rapid forgetting. _J. Exp. Child Psychol._ 91, 1–23 (2005). Article Google Scholar * Zimmer, H. D. Visual and spatial working memory: from boxes to
networks. _Neurosci. Biobehav. Rev._ 32, 1373–1395 (2008). Article PubMed Google Scholar * Visu-Petra, L., Benga, O., Tincas, I. & Miclea, M. Visual-spatial processing in children and
adolescents with Down's syndrome: a computerized assessment of memory skills. _J. Intellect. Disabil. Res._ 51, 942–952 (2007). Article CAS PubMed Google Scholar * Lanfranchi, S.,
Carretti, B., Spano, G. & Cornoldi, C. A specific deficit in visuospatial simultaneous working memory in Down syndrome. _J. Intellect. Disabil. Res._ 53, 474–483 (2009). Article CAS
PubMed Google Scholar * Colom, R., Jung, R. E. & Haier, R. J. General intelligence and memory span: evidence for a common neuroanatomic framework. _Cogn. Neuropsychol._ 24, 867–878
(2007). Article PubMed Google Scholar * Jarrold, C. & Towse, J. N. Individual differences in working memory. _Neuroscience_ 139, 39–50 (2006). Article CAS PubMed Google Scholar *
Nairne, J. S. Remembering over the short-term: the case against the standard model. _Annu. Rev. Psychol._ 53, 53–81 (2002). Article PubMed Google Scholar * Nash, H. & Heath, J. The
role of vocabulary, working memory and inference making ability in reading comprehension in Down syndrome. _Res. Dev. Disabil._ 32, 1782–1791 (2011). Article PubMed Google Scholar *
Saito, S., Jarrold, C. & Riby, D. M. Exploring the forgetting mechanisms in working memory: evidence from a reasoning span test. _Q. J. Exp. Psychol._ 62, 1401–1419 (2009). Article
Google Scholar * Lanfranchi, S., Jerman, O., Dal Pont, E., Alberti, A. & Vianello, R. Executive function in adolescents with Down Syndrome. _J. Intellect. Disabil. Res._ 54, 308–319
(2010). Article CAS PubMed Google Scholar * Lemons, C. J. & Fuchs, D. Phonological awareness of children with Down syndrome: its role in learning to read and the effectiveness of
related interventions. _Res. Dev. Disabil._ 31, 316–330 (2010). Article PubMed Google Scholar * Jarrold, C., Cocksey, J. & Dockerill, E. Phonological similarity and lexicality effects
in children's verbal short-term memory: concerns about the interpretation of probed recall data. _Q. J. Exp. Psychol._ 61, 324–340 (2008). Article Google Scholar * Fidler, D. J.,
Most, D. E. & Guiberson, M. M. Neuropsychological correlates of word identification in Down syndrome. _Res. Dev. Disabil._ 26, 487–501 (2005). Article PubMed Google Scholar * Jarrold,
C., Baddeley, A. D. & Phillips, C. E. Verbal short-term memory in Down syndrome: a problem of memory, audition, or speech? _J. Speech Lang. Hear. Res._ 45, 531–544 (2002). Article
PubMed Google Scholar * Brock, J. & Jarrold, C. Serial order reconstruction in Down syndrome: evidence for a selective deficit in verbal short-term memory. _J. Child Psychol.
Psychiatry_ 46, 304–316 (2005). Article PubMed Google Scholar * Robertson Ringenbach, S. D., Chua, R., Maraj, B. K., Kao, J. C. & Weeks, D. J. Bimanual coordination dynamics in adults
with Down syndrome. _Motor Control_ 6, 388–407 (2002). Article PubMed Google Scholar * Robertson, S. D., Van Gemmert, A. W. & Maraj, B. K. Auditory information is beneficial for
adults with Down syndrome in a continuous bimanual task. _Acta Psychol._ 110, 213–229 (2002). Article Google Scholar * Elliott, D., Weeks, D. J. & Gray, S. Manual and oral praxis in
adults with Down's syndrome. _Neuropsychologia_ 28, 1307–1315 (1990). Article CAS PubMed Google Scholar * Elliott, D. & Weeks, D. J. Cerebral specialization and the control of
oral and limb movements for individuals with Down's syndrome. _J. Mot Behav._ 22, 6–18 (1990). Article CAS PubMed Google Scholar * Welsh, T. N. & Elliott, D. Gender differences
in a dichotic listening and movement task: lateralization or strategy? _Neuropsychologia_ 39, 25–35 (2001). Article CAS PubMed Google Scholar * Brunamonti, E. et al. Cognitive control of
movement in Down syndrome. _Res. Dev. Disabil._ 32, 1792–1797 (2011). Article PubMed Google Scholar * Davis, W. E. & Kelso, J. A. Analysis of “invariant characteristics” in the motor
control of down's syndrome and normal subjects. _J. Mot. Behav._ 14, 194–212 (1982). Article CAS PubMed Google Scholar * Virji-Babul, N. et al. Altered brain dynamics during
voluntary movement in individuals with Down syndrome. _Neuroreport_ 22, 358–364 (2011). Article PubMed Google Scholar * Vicari, S., Bellucci, S. & Carlesimo, G. A. Implicit and
explicit memory: a functional dissociation in persons with Down syndrome. _Neuropsychologia_ 38, 240–251 (2000). Article CAS PubMed Google Scholar * Vakil, E. & Lifshitz-Zehavi, H.
Solving the Raven Progressive Matrices by adults with intellectual disability with/without Down syndrome: different cognitive patterns as indicated by eye-movements. _Res. Dev. Disabil._ 33,
645–654 (2012). Article PubMed Google Scholar * Pinter, J. D., Eliez, S., Schmitt, J. E., Capone, G. T. & Reiss, A. L. Neuroanatomy of Down's syndrome: a high-resolution MRI
study. _Am. J. Psychiatry_ 158, 1659–1665 (2001). A SEMINAL PAPER SHOWING THE ALTERED NEUROANATOMY IN PATIENTS WITH DOWN SYNDROME PATIENTS USING MRI. Article CAS PubMed Google Scholar *
Raz, N. et al. Selective neuroanatomic abnormalities in Down's syndrome and their cognitive correlates: evidence from MRI morphometry. _Neurology_ 45, 356–366 (1995). Article CAS
PubMed Google Scholar * Menghini, D., Costanzo, F. & Vicari, S. Relationship between brain and cognitive processes in Down syndrome. _Behav. Genet._ 41, 381–393 (2011). THIS WAS ONE OF
SEVERAL STUDIES THAT ATTEMPTED TO CORRELATE STRUCTURAL ABNORMALITIES WITH COGNITIVE EFFICIENCY. Article PubMed Google Scholar * Jernigan, T. L., Bellugi, U., Sowell, E., Doherty, S.
& Hesselink, J. R. Cerebral morphologic distinctions between Williams and Down syndromes. _Arch. Neurol._ 50, 186–191 (1993). Article CAS PubMed Google Scholar * Jarrold, C.,
Baddeley, A. D. & Phillips, C. Long-term memory for verbal and visual information in Down syndrome and Williams syndrome: performance on the Doors and People test. _Cortex_ 43, 233–247
(2007). Article PubMed Google Scholar * Vicari, S., Bellucci, S. & Carlesimo, G. A. Evidence from two genetic syndromes for the independence of spatial and visual working memory.
_Dev. Med. Child Neurol._ 48, 126–131 (2006). Article PubMed Google Scholar * Di Filippo, M. et al. Impaired plasticity at specific subset of striatal synapses in the Ts65Dn mouse model
of Down syndrome. _Biol. Psychiatry_ 67, 666–671 (2010). Article CAS PubMed Google Scholar * Tomporowski, P. D., Hayden, A. M. & Applegate, B. Effects of background event rate on
sustained attention of mentally retarded and nonretarded adults. _Am. J. Ment. Retard._ 94, 499–508 (1990). CAS PubMed Google Scholar * Rigoldi, C. et al. Gait analysis and cerebral
volumes in Down's syndrome. _Funct. Neurol._ 24, 147–152 (2009). CAS PubMed Google Scholar * Jacola, L. M. et al. Functional magnetic resonance imaging of cognitive processing in
young adults with Down syndrome. _Am. J. Intellect. Dev. Disabil._ 116, 344–359 (2011). Article PubMed Google Scholar * Colom, R., Karama, S., Jung, R. E. & Haier, R. J. Human
intelligence and brain networks. _Dialogues Clin. Neurosci._ 12, 489–501 (2010). PubMed PubMed Central Google Scholar * Fabbro, F., Libera, L. & Tavano, A. A callosal transfer deficit
in children with developmental language disorder. _Neuropsychologia_ 40, 1541–1546 (2002). Article PubMed Google Scholar * Pennington, B. F., Moon, J., Edgin, J., Stedron, J. &
Nadel, L. The neuropsychology of Down syndrome: evidence for hippocampal dysfunction. _Child Dev._ 74, 75–93 (2003). A SEMINAL PAPER SHOWING HIPPOCAMPAL DYSFUNCTION IN PATIENTS WITH DOWN
SYNDROME THAT WAS REVEALED USING SPECIFIC NEUROPSYCHOLOGICAL TESTS THAT WERE BASED ON MOUSE WORK. Article PubMed Google Scholar * Sturgeon, X. & Gardiner, K. J. Transcript catalogs of
human chromosome 21 and orthologous chimpanzee and mouse regions. _Mamm. Genome_ 22, 261–271 (2011). Article PubMed Google Scholar * Delabar, J. M. et al. Molecular mapping of
twenty-four features of Down syndrome on chromosome 21. _Eur. J. Hum. Genet._ 1, 114–124 (1993). Article CAS PubMed Google Scholar * Korenberg, J. R. et al. Down syndrome phenotypes: the
consequences of chromosomal imbalance. _Proc. Natl Acad. Sci. USA_ 91, 4997–5001 (1994). Article CAS PubMed PubMed Central Google Scholar * Korbel, J. O. et al. The genetic
architecture of Down syndrome phenotypes revealed by high-resolution analysis of human segmental trisomies. _Proc. Natl Acad. Sci. USA_ 106, 12031–12036 (2009). THIS STUDY SHOWS THAT
DIFFERENT REGIONS OF HSA21 CONTRIBUTE TO DIFFERENT DISEASE-RELATED PHENOTYPES, ARGUING AGAINST A SINGLE CRITICAL REGION IN HSA21 (ALSO SEE REFERENCE 57). Article PubMed PubMed Central
Google Scholar * Lyle, R. et al. Genotype–phenotype correlations in Down syndrome identified by array CGH in 30 cases of partial trisomy and partial monosomy chromosome 21. _Eur. J. Hum.
Genet._ 17, 454–466 (2009). Article CAS PubMed Google Scholar * Dierssen, M. et al. Functional genomics of Down syndrome: a multidisciplinary approach. _J. Neural Transm. Suppl._ 61,
131–148 (2001). Google Scholar * Pritchard, M., Reeves, R. H., Dierssen, M., Patterson, D. & Gardiner, K. J. Down syndrome and the genes of human chromosome 21: current knowledge and
future potentials. Report on the Expert workshop on the biology of chromosome 21 genes: towards gene-phenotype correlations in Down syndrome. Washington D.C., September 28-October 1, 2007.
_Cytogenet. Genome Res._ 121, 67–77 (2008). Article CAS PubMed Google Scholar * Pritchard, M. A. & Kola, I. The “gene dosage effect” hypothesis versus the “amplified developmental
instability” hypothesis in Down syndrome. _J. Neural Transm. Suppl._ 57, 293–303 (1999). CAS PubMed Google Scholar * Wiseman, F. K., Alford, K. A., Tybulewicz, V. L. & Fisher, E. M.
Down syndrome—recent progress and future prospects. _Hum. Mol. Genet._ 18, R75–R83 (2009). Article CAS PubMed PubMed Central Google Scholar * Toiber, D. et al. Engineering _DYRK1A_
overdosage yields Down syndrome-characteristic cortical splicing aberrations. _Neurobiol. Dis._ 40, 348–359 (2010). THIS STUDY SHOWS THAT A DOWN SYNDROME CANDIDATE GENE ON HSA21, _DYRK1A_ ,
HAS MASTER REGULATORY FUNCTIONS IN TRANSCRIPTION AND MRNA SPLICING AND THUS MAY EXERT WIDE-RANGING EFFECTS, ACTING ON GENES THAT ARE FOUND ON HSA21 AND OTHER CHROMOSOMES. ALSO SEE REFERENCES
63–65. Article CAS PubMed Google Scholar * Kahlem, P. et al. Transcript level alterations reflect gene dosage effects across multiple tissues in a mouse model of down syndrome. _Genome
Res._ 14, 1258–1267 (2004). THIS STUDY TESTED THE HYPOTHESIS THAT HSA21 TRANSCRIPTS ARE OVEREXPRESSED BY ABOUT 50% IN TRISOMIC CELLS. Article CAS PubMed PubMed Central Google Scholar *
Arron, J. R. et al. NFAT dysregulation by increased dosage of _DSCR1_ and _DYRK1A_ on chromosome 21. _Nature_ 441, 595–600 (2006). DYRK1A PHOSPHORYLATES NFAT WHILE CALCIPRESSIN 1
PHOSPHORYLATION AT THR192 BY DYRK1A ENHANCES THE ABILITY OF CALCIPRESSIN 1 TO INHIBIT THE PHOSPHATASE ACTIVITY OF CALCINEURIN, LEADING TO REDUCED NFAT TRANSCRIPTIONAL ACTIVITY. Article CAS
PubMed Google Scholar * Vilardell, M. et al. Meta-analysis of heterogeneous Down Syndrome data reveals consistent genome-wide dosage effects related to neurological processes. _BMC
Genomics_ 12, 229 (2011). Article PubMed PubMed Central Google Scholar * Ferrando-Miguel, R., Cheon, M. S., Yang, J. W. & Lubec, G. Overexpression of transcription factor BACH1 in
fetal Down syndrome brain. _J. Neural Transm. Suppl._ 67, 193–205 (2003). Article CAS Google Scholar * Shim, K. S., Ferrando-Miguel, R. & Lubec, G. Aberrant protein expression of
transcription factors BACH1 and ERG, both encoded on chromosome 21, in brains of patients with Down syndrome and Alzheimer's disease. _J. Neural Transm. Suppl._ 67, 39–49 (2003).
Article CAS Google Scholar * Osato, M. & Ito, Y. Increased dosage of the _RUNX1/AML1_ gene: a third mode of RUNX leukemia? _Crit. Rev. Eukaryot. Gene Expr._ 15, 217–228 (2005).
Article CAS PubMed Google Scholar * Altafaj, X. et al. Neurodevelopmental delay, motor abnormalities and cognitive deficits in transgenic mice overexpressing Dyrk1A (minibrain), a murine
model of Down's syndrome. _Hum. Mol. Genet._ 10, 1915–1923 (2001). _DYRK1A_ IS A MAJOR CANDIDATE GENE FOR DOWN SYNDROME. THIS PAPER REPORTS THE CONSTRUCTION OF THE FIRST TRANSGENIC
MOUSE MODEL OVEREXPRESSING THIS KINASE. MANY OTHER DYRK1A OVEREXPRESSION MODELS HAVE SUBSEQUENTLY BEEN CREATED. Article CAS PubMed Google Scholar * Ahn, K. J. et al. DYRK1A BAC
transgenic mice show altered synaptic plasticity with learning and memory defects. _Neurobiol. Dis._ 22, 463–472 (2006). Article CAS PubMed Google Scholar * Ferrer, I. et al.
Constitutive Dyrk1A is abnormally expressed in Alzheimer disease, Down syndrome, Pick disease, and related transgenic models. _Neurobiol. Dis._ 20, 392–400 (2005). THIS WAS ONE OF THE FIRST
PAPERS SUGGESTING A ROLE FOR DYRK1A IN ALZHEIMER'S DISEASE. Article CAS PubMed Google Scholar * Fuentes, J. J. et al. A new human gene from the Down syndrome critical region encodes
a proline-rich protein highly expressed in fetal brain and heart. _Hum. Mol. Genet._ 4, 1935–1944 (1995). Article CAS PubMed Google Scholar * Shapiro, B. L. Amplified developmental
instability in Down's syndrome. _Ann. Hum. Genet._ 38, 429–437 (1975). THE AMPLIFIED DEVELOPMENTAL INSTABILITY HYPOTHESIS, WHICH IS PROPOSED IN THIS PAPER, STATES THAT THE DOSAGE
IMBALANCE OF HSA21 LEADS TO A NON-SPECIFIC DISTURBANCE OF CELLULAR HOMEOSTASIS. Article CAS PubMed Google Scholar * Shapiro, B. L. Developmental instability of the cerebellum and its
relevance to Down syndrome. _J. Neural Transm. Suppl._ 61, 11–34 (2001). Google Scholar * Olson, L. E. et al. Down syndrome mouse models Ts65Dn, Ts1Cje, and Ms1Cje/Ts65Dn exhibit variable
severity of cerebellar phenotypes. _Dev. Dyn._ 230, 581–589 (2004). THIS PAPER MADE AN IMPORTANT CONTRIBUTION TO THE UNDERSTANDING OF DOWN SYNDROME CEREBELLAR PHENOTYPES. THE CEREBELLAR
ALTERATIONS IN DOWN SYNDROME WERE THOUGHT TO PARTICIPATE MAINLY IN MOTOR PHENOTYPES, BUT ABNORMALITIES IN THIS REGION ARE NOW THOUGHT TO ALSO AFFECT COGNITIVE PROCESSES. Article CAS PubMed
Google Scholar * Yu, T. et al. Effects of individual segmental trisomies of human chromosome 21 syntenic regions on hippocampal long-term potentiation and cognitive behaviors in mice.
_Brain Res._ 1366, 162–171 (2010). Article CAS PubMed PubMed Central Google Scholar * Kuhn, D. E. et al. Human chromosome 21-derived miRNAs are overexpressed in down syndrome brains and
hearts. _Biochem. Biophys. Res. Commun._ 370, 473–477 (2008). THIS STUDY SHOWED THAT HSA21-DERIVED MICRORNAS ARE OVEREXPRESSED IN DOWN SYNDROME BRAIN AND HEART SPECIMENS, LEADING TO
IMPROPER REPRESSION OF SPECIFIC TARGET PROTEINS THAT ARE LINKED TO SPECIFIC PHENOTYPES. Article CAS PubMed PubMed Central Google Scholar * Kuhn, D. E. et al. Chromosome 21-derived
microRNAs provide an etiological basis for aberrant protein expression in human Down syndrome brains. _J. Biol. Chem._ 285, 1529–1543 (2010). Article CAS PubMed Google Scholar * Loudin,
M. G. et al. Genomic profiling in Down syndrome acute lymphoblastic leukemia identifies histone gene deletions associated with altered methylation profiles. _Leukemia_ 25, 1555–1563 (2011).
Article CAS PubMed PubMed Central Google Scholar * Pletcher, M. T., Wiltshire, T., Cabin, D. E., Villanueva, M. & Reeves, R. H. Use of comparative physical and sequence mapping to
annotate mouse chromosome 16 and human chromosome 21. _Genomics_ 74, 45–54 (2001). Article CAS PubMed Google Scholar * Davisson, M. T. et al. Segmental trisomy as a mouse model for Down
syndrome. _Prog. Clin. Biol. Res._ 384, 117–133 (1993). THE CREATION OF THE FIRST VIABLE TRISOMIC MOUSE (TS65DN) WAS A REVOLUTION IN THE FIELD OF DOWN SYNDROME RESEARCH AND PROVIDED THE BEST
_IN VIVO_ MODEL FOR DOWN SYNDROME. ALSO SEE REFERENCE 82. CAS PubMed Google Scholar * Reeves, R. H. et al. A mouse model for Down syndrome exhibits learning and behaviour deficits.
_Nature Genet._ 11, 177–184 (1995). Article CAS PubMed Google Scholar * Dierssen, M. et al. Alterations of neocortical pyramidal cell phenotype in the Ts65Dn mouse model of Down
syndrome: effects of environmental enrichment. _Cereb. Cortex_ 13, 758–764 (2003). Article CAS PubMed Google Scholar * Sago, H. et al. Ts1Cje, a partial trisomy 16 mouse model for Down
syndrome, exhibits learning and behavioral abnormalities. _Proc. Natl Acad. Sci. USA_ 95, 6256–6261 (1998). Article CAS PubMed PubMed Central Google Scholar * Villar, A. J. et al.
Identification and characterization of a new Down syndrome model, Ts[Rb(12.1716)]2Cje, resulting from a spontaneous Robertsonian fusion between T(171)65Dn and mouse chromosome 12. _Mamm.
Genome_ 16, 79–90 (2005). Article CAS PubMed Google Scholar * Ishihara, K. et al. Enlarged brain ventricles and impaired neurogenesis in the Ts1Cje and Ts2Cje mouse models of Down
syndrome. _Cereb. Cortex_ 20, 1131–1143 (2010). Article PubMed Google Scholar * Olson, L. E. et al. Trisomy for the Down syndrome 'critical region' is necessary but not
sufficient for brain phenotypes of trisomic mice. _Hum. Mol. Genet._ 16, 774–782 (2007). THIS STUDY EXPLORED THE ROLE OF THE DSCR AND PRESENTED EVIDENCE THAT QUESTIONED THE DSCR HYPOTHESIS.
Article CAS PubMed Google Scholar * Belichenko, N. P. et al. The “Down syndrome critical region” is sufficient in the mouse model to confer behavioral, neurophysiological, and synaptic
phenotypes characteristic of Down syndrome. _J. Neurosci._ 29, 5938–5948 (2009). Article CAS PubMed PubMed Central Google Scholar * Olson, L. E., Richtsmeier, J. T., Leszl, J. &
Reeves, R. H. A chromosome 21 critical region does not cause specific Down syndrome phenotypes. _Science_ 306, 687–690 (2004). Article CAS PubMed PubMed Central Google Scholar *
Aldridge, K., Reeves, R. H., Olson, L. E. & Richtsmeier, J. T. Differential effects of trisomy on brain shape and volume in related aneuploid mouse models. _Am. J. Med. Genet. A_ 143A,
1060–1070 (2007). Article PubMed PubMed Central Google Scholar * Duchon, A. et al. The telomeric part of the human chromosome 21 from _Cstb_ to _Prmt2_ is not necessary for the locomotor
and short-term memory deficits observed in the Tc1 mouse model of Down syndrome. _Behav. Brain Res._ 217, 271–281 (2011). Article CAS PubMed PubMed Central Google Scholar *
O'Doherty, A. et al. An aneuploid mouse strain carrying human chromosome 21 with Down syndrome phenotypes. _Science_ 309, 2033–2037 (2005). THIS PAPER DETAILS THE CREATION OF THE FIRST
'HUMANIZED' DOWN SYNDROME MOUSE MODEL. Article CAS PubMed PubMed Central Google Scholar * Morice, E. et al. Preservation of long-term memory and synaptic plasticity despite
short-term impairments in the Tc1 mouse model of Down syndrome. _Learn. Mem._ 15, 492–500 (2008). Article CAS PubMed PubMed Central Google Scholar * Galante, M. et al. Impairments in
motor coordination without major changes in cerebellar plasticity in the Tc1 mouse model of Down syndrome. _Hum. Mol. Genet._ 18, 1449–1463 (2009). Article CAS PubMed PubMed Central
Google Scholar * Reynolds, T. The triple test as a screening technique for Down syndrome: reliability and relevance. _Int. J. Womens Health_ 2, 83–88 (2010). Article CAS PubMed PubMed
Central Google Scholar * Li, Z. et al. Duplication of the entire 22.9 Mb human chromosome 21 syntenic region on mouse chromosome 16 causes cardiovascular and gastrointestinal
abnormalities. _Hum. Mol. Genet._ 16, 1359–1366 (2007). Article CAS PubMed Google Scholar * Belichenko, P. V., Kleschevnikov, A. M., Salehi, A., Epstein, C. J. & Mobley, W. C.
Synaptic and cognitive abnormalities in mouse models of Down syndrome: exploring genotype-phenotype relationships. _J. Comp. Neurol._ 504, 329–345 (2007). Article CAS PubMed Google
Scholar * Escorihuela, R. M. et al. A behavioral assessment of Ts65Dn mice: a putative Down syndrome model. _Neurosci. Lett._ 199, 143–146 (1995). Article CAS PubMed Google Scholar *
Nambu, J. R., Lewis, J. O., Wharton, K. A. Jr & Crews, S. T. The _Drosophila_ single-minded gene encodes a helix-loop-helix protein that acts as a master regulator of CNS midline
development. _Cell_ 67, 1157–1167 (1991). Article CAS PubMed Google Scholar * Rachidi, M. et al. Spatial and temporal localization during embryonic and fetal human development of the
transcription factor _SIM2_ in brain regions altered in Down syndrome. _Int. J. Dev. Neurosci._ 23, 475–484 (2005). Article CAS PubMed Google Scholar * Chrast, R. et al. Mice trisomic
for a bacterial artificial chromosome with the single-minded 2 gene (_Sim2_) show phenotypes similar to some of those present in the partial trisomy 16 mouse models of Down syndrome. _Hum.
Mol. Genet._ 9, 1853–1864 (2000). Article CAS PubMed Google Scholar * Chrast, R. et al. The mouse brain transcriptome by SAGE: differences in gene expression between P30 brains of the
partial trisomy 16 mouse model of Down syndrome (Ts65Dn) and normals. _Genome Res._ 10, 2006–2021 (2000). Article CAS PubMed PubMed Central Google Scholar * Harashima, C. et al.
Elevated expression of the G-protein-activated inwardly rectifying potassium channel 2 (_GIRK2_) in cerebellar unipolar brush cells of a Down syndrome mouse model. _Cell. Mol. Neurobiol._
26, 719–734 (2006). Article CAS PubMed Google Scholar * Cooper, A. et al. Trisomy of the G protein-coupled K+ channel gene, _Kcnj6_, affects reward mechanisms, cognitive functions, and
synaptic plasticity in mice. _Proc. Natl Acad. Sci. USA_ 109, 2642–2647 (2012). Article PubMed PubMed Central Google Scholar * Alves-Sampaio, A., Troca-Marin, J. A. & Montesinos, M.
L. NMDA-mediated regulation of DSCAM dendritic local translation is lost in a mouse model of Down's syndrome. _J. Neurosci._ 30, 13537–13548 (2010). Article CAS PubMed PubMed Central
Google Scholar * Gotti, S., Caricati, E. & Panzica, G. Alterations of brain circuits in Down syndrome murine models. _J. Chem. Neuroanat._ 42, 317–326 (2011). Article CAS PubMed
Google Scholar * Liu, C. et al. Mouse models for down syndrome-associated developmental cognitive disabilities. _Dev. Neurosci._ 33, 404–413 (2011). Article CAS PubMed PubMed Central
Google Scholar * Dierssen, M. et al. Murine models for Down syndrome. _Physiol. Behav._ 73, 859–871 (2001). Article CAS PubMed Google Scholar * Dierssen, M. & de Lagran, M. M.
DYRK1A (dual-specificity tyrosine-phosphorylated and -regulated kinase 1A): a gene with dosage effect during development and neurogenesis. _ScientificWorldJournal_ 6, 1911–1922 (2006).
Article CAS PubMed PubMed Central Google Scholar * Smith, D. J., Zhu, Y., Zhang, J., Cheng, J. F. & Rubin, E. M. Construction of a panel of transgenic mice containing a contiguous
2-Mb set of YAC/P1 clones from human chromosome 21q22.2. _Genomics_ 27, 425–434 (1995). SMITH _ET AL_ . CREATED THE FIRST _IN VIVO_ LIBRARY OF MICE BEARING CONTIGUOUS PARTIAL TRISOMIES.
Article CAS PubMed Google Scholar * Guedj, F. et al. DYRK1A: a master regulatory protein controlling brain growth. _Neurobiol. Dis._ 46, 190–203 (2012). Article CAS PubMed Google
Scholar * Smith, D. J. & Rubin, E. M. Functional screening and complex traits: human 21q22.2 sequences affecting learning in mice. _Hum. Mol. Genet._ 6, 1729–1733 (1997). Article CAS
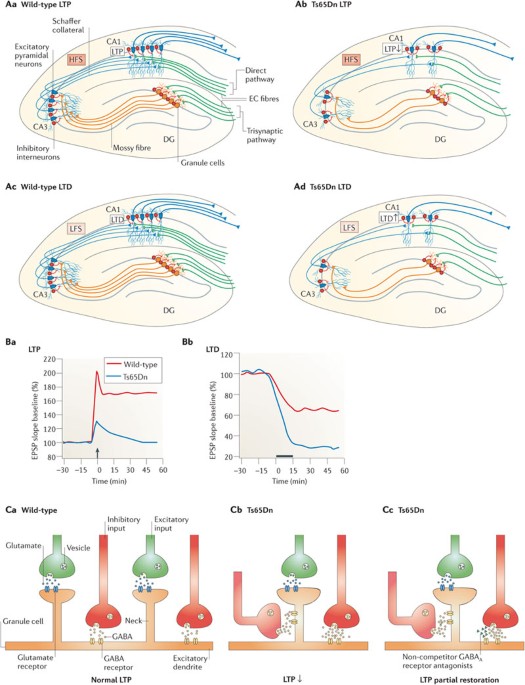
PubMed Google Scholar * Kleschevnikov, A. M. et al. Hippocampal long-term potentiation suppressed by increased inhibition in the Ts65Dn mouse, a genetic model of Down syndrome. _J.
Neurosci._ 24, 8153–8160 (2004). Article CAS PubMed PubMed Central Google Scholar * Yuste, R. Dendritic spines and distributed circuits. _Neuron_ 71, 772–781 (2011). Article CAS
PubMed PubMed Central Google Scholar * Dierssen, M. & Ramakers, G. J. Dendritic pathology in mental retardation: from molecular genetics to neurobiology. _Genes Brain Behav._ 5, 48–60
(2006). Article CAS PubMed Google Scholar * Galdzicki, Z. & Siarey, R. J. Understanding mental retardation in Down's syndrome using trisomy 16 mouse models. _Genes Brain
Behav._ 2, 167–178 (2003). Article CAS PubMed Google Scholar * Siarey, R. J., Stoll, J., Rapoport, S. I. & Galdzicki, Z. Altered long-term potentiation in the young and old Ts65Dn
mouse, a model for Down Syndrome. _Neuropharmacology_ 36, 1549–1554 (1997). Article CAS PubMed Google Scholar * Kurt, M. A., Davies, D. C., Kidd, M., Dierssen, M. & Florez, J.
Synaptic deficit in the temporal cortex of partial trisomy 16 (Ts65Dn) mice. _Brain Res._ 858, 191–197 (2000). THIS IS THE FIRST PAPER DESCRIBING THE EXCITATION–INHIBITION IMBALANCE IN THE
HIPPOCAMPUS OF A DOWN SYNDROME TRISOMIC MOUSE MODEL (TS65DN). Article CAS PubMed Google Scholar * Pereira, P. L. et al. A new mouse model for the trisomy of the _Abcg1−U2af1_ region
reveals the complexity of the combinatorial genetic code of down syndrome. _Hum. Mol. Genet._ 18, 4756–4769 (2009). Article CAS PubMed PubMed Central Google Scholar * Martinez de
Lagran, M. et al. Dyrk1A influences neuronal morphogenesis through regulation of cytoskeletal dynamics in mammalian cortical neurons. _Cereb. Cortex_ 2 Jan 2012 (doi:10.1093/cercor/bhr362).
* Kurt, M. A., Kafa, M. I., Dierssen, M. & Davies, D. C. Deficits of neuronal density in CA1 and synaptic density in the dentate gyrus, CA3 and CA1, in a mouse model of Down syndrome.
_Brain Res._ 1022, 101–109 (2004). Article CAS PubMed Google Scholar * Belichenko, P. V. et al. Excitatory-inhibitory relationship in the fascia dentata in the Ts65Dn mouse model of Down
syndrome. _J. Comp. Neurol._ 512, 453–466 (2009). Article PubMed PubMed Central Google Scholar * Siarey, R. J. et al. Increased synaptic depression in the Ts65Dn mouse, a model for
mental retardation in Down syndrome. _Neuropharmacology_ 38, 1917–1920 (1999). Article CAS PubMed Google Scholar * Popov, V. I., Kleschevnikov, A. M., Klimenko, O. A., Stewart, M. G.
& Belichenko, P. V. Three-dimensional synaptic ultrastructure in the dentate gyrus and hippocampal area CA3 in the Ts65Dn mouse model of Down syndrome. _J. Comp. Neurol._ 519, 1338–1354
(2011). Article CAS PubMed Google Scholar * Escorihuela, R. M. et al. Early environmental stimulation produces long-lasting changes on β-adrenoceptor transduction system. _Neurobiol.
Learn. Mem._ 64, 49–57 (1995). Article CAS PubMed Google Scholar * Coussons-Read, M. E. & Crnic, L. S. Behavioral assessment of the Ts65Dn mouse, a model for Down syndrome: altered
behavior in the elevated plus maze and open field. _Behav. Genet._ 26, 7–13 (1996). Article CAS PubMed Google Scholar * Hyde, L. A., Crnic, L. S., Pollock, A. & Bickford, P. C. Motor
learning in Ts65Dn mice, a model for Down syndrome. _Dev. Psychobiol._ 38, 33–45 (2001). Article CAS PubMed Google Scholar * Driscoll, L. L. et al. Impaired sustained attention and
error-induced stereotypy in the aged Ts65Dn mouse: a mouse model of Down syndrome and Alzheimer's disease. _Behav. Neurosci._ 118, 1196–1205 (2004). Article PubMed Google Scholar *
Escorihuela, R. M. et al. Impaired short- and long-term memory in Ts65Dn mice, a model for Down syndrome. _Neurosci. Lett._ 247, 171–174 (1998). Article CAS PubMed Google Scholar *
Martinez-Cue, C. et al. Differential effects of environmental enrichment on behavior and learning of male and female Ts65Dn mice, a model for Down syndrome. _Behav. Brain Res._ 134, 185–200
(2002). Article PubMed Google Scholar * Baamonde, C., Martinez-Cue, C., Florez, J. & Dierssen, M. G-protein-associated signal transduction processes are restored after postweaning
environmental enrichment in Ts65Dn, a Down Syndrome mouse model. _Dev. Neurosci._ 33, 442–450 (2011). Article CAS PubMed Google Scholar * Casanova, M. F., Walker, L. C., Whitehouse, P.
J. & Price, D. L. Abnormalities of the nucleus basalis in Down's syndrome. _Ann. Neurol._ 18, 310–313 (1985). Article CAS PubMed Google Scholar * Mann, D. M., Yates, P. O. &
Hawkes, J. The pathology of the human locus ceruleus. _Clin. Neuropathol._ 2, 1–7 (1983). CAS PubMed Google Scholar * Salehi, A. et al. Restoration of norepinephrine-modulated contextual
memory in a mouse model of Down syndrome. _Sci. Transl. Med._ 1, 7ra17 (2009). Article CAS PubMed Google Scholar * Chang, Q. & Gold, P. E. Age-related changes in memory and in
acetylcholine functions in the hippocampus in the Ts65Dn mouse, a model of Down syndrome. _Neurobiol. Learn. Mem._ 89, 167–177 (2008). Article CAS PubMed Google Scholar * Rueda, N.,
Florez, J. & Martinez-Cue, C. Effects of chronic administration of SGS-111 during adulthood and during the pre- and post-natal periods on the cognitive deficits of Ts65Dn mice, a model
of Down syndrome. _Behav. Brain Res._ 188, 355–367 (2008). Article CAS PubMed Google Scholar * Holtzman, D. M. et al. Developmental abnormalities and age-related neurodegeneration in a
mouse model of Down syndrome. _Proc. Natl Acad. Sci. USA_ 93, 13333–13338 (1996). Article CAS PubMed PubMed Central Google Scholar * Prasher, V. P., Fung, N. & Adams, C.
Rivastigmine in the treatment of dementia in Alzheimer's disease in adults with Down syndrome. _Int. J. Geriatr. Psychiatry_ 20, 496–497 (2005). Article CAS PubMed Google Scholar *
Van der Molen, M. J. et al. Attentional set-shifting in fragile X syndrome. _Brain Cogn._ 78, 206–217 (2012). Article CAS PubMed Google Scholar * Hanson, J. E., Blank, M., Valenzuela, R.
A., Garner, C. C. & Madison, D. V. The functional nature of synaptic circuitry is altered in area CA3 of the hippocampus in a mouse model of Down's syndrome. _J. Physiol._ 579,
53–67 (2007). Article CAS PubMed Google Scholar * Fagiolini, M. & Hensch, T. K. Inhibitory threshold for critical-period activation in primary visual cortex. _Nature_ 404, 183–186
(2000). Article CAS PubMed Google Scholar * Southwell, D. G., Froemke, R. C., Alvarez-Buylla, A., Stryker, M. P. & Gandhi, S. P. Cortical plasticity induced by inhibitory neuron
transplantation. _Science_ 327, 1145–1148 (2010). Article CAS PubMed PubMed Central Google Scholar * Fernandez, F. et al. Pharmacotherapy for cognitive impairment in a mouse model of
Down syndrome. _Nature Neurosci._ 10, 411–413 (2007). Article CAS PubMed Google Scholar * Rueda, N., Florez, J. & Martinez-Cue, C. Chronic pentylenetetrazole but not donepezil
treatment rescues spatial cognition in Ts65Dn mice, a model for Down syndrome. _Neurosci. Lett._ 433, 22–27 (2008). Article CAS PubMed Google Scholar * Owens, D. F. & Kriegstein, A.
R. Developmental neurotransmitters? _Neuron_ 36, 989–991 (2002). Article CAS PubMed Google Scholar * Baroncelli, L. et al. Brain plasticity and disease: a matter of inhibition. _Neural
Plast._ 2011, 286073 (2011). Article PubMed PubMed Central Google Scholar * Owens, D. F. & Kriegstein, A. R. Is there more to GABA than synaptic inhibition? _Nature Rev. Neurosci._
3, 715–727 (2002). Article CAS Google Scholar * Becker, L., Mito, T., Takashima, S. & Onodera, K. Growth and development of the brain in Down syndrome. _Prog. Clin. Biol. Res._ 373,
133–152 (1991). CAS PubMed Google Scholar * Braudeau, J. et al. Specific targeting of the GABA-A receptor α5 subtype by a selective inverse agonist restores cognitive deficits in Down
syndrome mice. _J. Psychopharmacol._ 25, 1030–1042 (2011). Article CAS PubMed PubMed Central Google Scholar * Costa, A. C., Scott-McKean, J. J. & Stasko, M. R. Acute injections of
the NMDA receptor antagonist memantine rescue performance deficits of the Ts65Dn mouse model of Down syndrome on a fear conditioning test. _Neuropsychopharmacology_ 33, 1624–1632 (2008).
Article CAS PubMed Google Scholar * Lockrow, J., Boger, H., Bimonte-Nelson, H. & Granholm, A. C. Effects of long-term memantine on memory and neuropathology in Ts65Dn mice, a model
for Down syndrome. _Behav. Brain Res._ 221, 610–622 (2011). Article CAS PubMed Google Scholar * Rueda, N. et al. Memantine normalizes several phenotypic features in the Ts65Dn mouse
model of Down syndrome. _J. Alzheimers Dis._ 21, 277–290 (2010). Article CAS PubMed Google Scholar * Marvanova, M. et al. The neuroprotective agent memantine induces brain-derived
neurotrophic factor and trkB receptor expression in rat brain. _Mol. Cell. Neurosci._ 18, 247–258 (2001). Article CAS PubMed Google Scholar * Westmark, C. J. et al. Reversal of fragile X
phenotypes by manipulation of AβPP/Aβ levels in _Fmr1_KO mice. _PLoS ONE_ 6, e26549 (2011). Article CAS PubMed PubMed Central Google Scholar * Lorenzi, H. A. & Reeves, R. H.
Hippocampal hypocellularity in the Ts65Dn mouse originates early in development. _Brain Res._ 1104, 153–159 (2006). Article CAS PubMed Google Scholar * Bianchi, P. et al. Early
pharmacotherapy restores neurogenesis and cognitive performance in the Ts65Dn mouse model for Down syndrome. _J. Neurosci._ 30, 8769–8779 (2010). Article CAS PubMed PubMed Central Google
Scholar * Roper, R. J. et al. Defective cerebellar response to mitogenic Hedgehog signaling in Down [corrected] syndrome mice. _Proc. Natl Acad. Sci. USA_ 103, 1452–1456 (2006). Article
CAS PubMed PubMed Central Google Scholar * Delcroix, J. D. et al. Trafficking the NGF signal: implications for normal and degenerating neurons. _Prog. Brain Res._ 146, 3–23 (2004). CAS
PubMed Google Scholar * Salehi, A., Faizi, M., Belichenko, P. V. & Mobley, W. C. Using mouse models to explore genotype-phenotype relationship in Down syndrome. _Ment. Retard. Dev.
Disabil. Res. Rev._ 13, 207–214 (2007). Article PubMed Google Scholar * Tejedor, F. J. & Hammerle, B. MNB/DYRK1A as a multiple regulator of neuronal development. _FEBS J._ 278,
223–235 (2011). Article CAS PubMed Google Scholar * Hammerle, B. et al. Transient expression of _Mnb/Dyrk1a_ couples cell cycle exit and differentiation of neuronal precursors by
inducing _p27_KIP1 expression and suppressing NOTCH signaling. _Development_ 138, 2543–2554 (2011). Article CAS PubMed PubMed Central Google Scholar * Yabut, O., Domogauer, J. &
D'Arcangelo, G. Dyrk1A overexpression inhibits proliferation and induces premature neuronal differentiation of neural progenitor cells. _J. Neurosci._ 30, 4004–4014 (2010). Article CAS
PubMed PubMed Central Google Scholar * Ortiz-Abalia, J. et al. Targeting Dyrk1A with AAVshRNA attenuates motor alterations in TgDyrk1A, a mouse model of Down syndrome. _Am. J. Hum.
Genet._ 83, 479–488 (2008). Article CAS PubMed PubMed Central Google Scholar * Guedj, F. et al. Green tea polyphenols rescue of brain defects induced by overexpression of DYRK1A. _PLoS
ONE_ 4, e4606 (2009). Article CAS PubMed PubMed Central Google Scholar * Gockler, N. et al. Harmine specifically inhibits protein kinase DYRK1A and interferes with neurite formation.
_FEBS J._ 276, 6324–6337 (2009). Article CAS PubMed Google Scholar * Mazur-Kolecka, B. et al. Effect of DYRK1A activity inhibition on development of neuronal progenitors isolated from
Ts65Dn mice. _J. Neurosci. Res._ 90, 999–1010 (2012). Article CAS PubMed Google Scholar * Kamenetz, F. et al. APP processing and synaptic function. _Neuron_ 37, 925–937 (2003). Article
CAS PubMed Google Scholar * Netzer, W. J. et al. Lowering β-amyloid levels rescues learning and memory in a Down syndrome mouse model. _PLoS ONE_ 5, e10943 (2010). Article CAS PubMed
PubMed Central Google Scholar * Chakrabarti, L. et al. _Olig1_ and _Olig2_ triplication causes developmental brain defects in Down syndrome. _Nature Neurosci._ 13, 927–934 (2010). THE
AUTHORS IDENTIFIED OLIG1 AND OLIG2 AS IMPORTANT PLAYERS IN THE DEVELOPMENTAL ALTERATIONS, LEADING TO INCREASED INHIBITION IN DOWN SYNDROME. Article CAS PubMed Google Scholar * Haydar, T.
F. & Reeves, R. H. Trisomy 21 and early brain development. _Trends Neurosci._ 35, 81–91 (2012). Article CAS PubMed Google Scholar * Edgin, J. O. et al. Development and validation of
the Arizona Cognitive Test Battery for Down syndrome. _J. Neurodev Disord._ 2, 149–164 (2010). THE ARIZONA COGNITIVE TEST BATTERY IS THE FIRST SERIOUS ATTEMPT TO CREATE A NEUROPSYCHOLOGICAL
EXPLORATION SCREEN THAT IS SPECIFIC FOR DOWN SYNDROME. Article PubMed PubMed Central Google Scholar * Nadel, L. Down's syndrome: a genetic disorder in biobehavioral perspective.
_Genes Brain Behav._ 2, 156–166 (2003). Article CAS PubMed Google Scholar * Ponting, C. P. & Belgard, T. G. Transcribed dark matter: meaning or myth? _Hum. Mol. Genet._ 19, R162–R168
(2010). Article CAS PubMed PubMed Central Google Scholar * Elton, T. S., Sansom, S. E. & Martin, M. M. Trisomy-21 gene dosage over-expression of miRNAs results in the
haploinsufficiency of specific target proteins. _RNA Biol._ 7, 540–547 (2010). Article CAS PubMed PubMed Central Google Scholar * Senti, K. A. & Brennecke, J. The piRNA pathway: a
fly's perspective on the guardian of the genome. _Trends Genet._ 26, 499–509 (2010). Article CAS PubMed PubMed Central Google Scholar * Singh Sandhu, K., Li, G., Sung, W. K. &
Ruan, Y. Chromatin interaction networks and higher order architectures of eukaryotic genomes. _J. Cell. Biochem._ 112, 2218–2221 (2011). Article CAS PubMed Google Scholar * Reinholdt, L.
G. et al. Meiotic behavior of aneuploid chromatin in mouse models of Down syndrome. _Chromosoma_ 118, 723–736 (2009). Article CAS PubMed PubMed Central Google Scholar * Cramer, N.
& Galdzicki, Z. From abnormal hippocampal synaptic plasticity in down syndrome mouse models to cognitive disability in down syndrome. _Neural Plast._ 2012, 101542 (2012). Article PubMed
PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This Review is dedicated to all people with Down syndrome. I also dedicate it to M. Flórez and E. Bishop, and I offer a
special thank you to J. Flórez, who is the professor that directed me to the field of Down syndrome research. I thank M. Martínez de Lagrán, G. Azkona and G. Arqué for their contributions
to putting together the movies, and D. D'Amico for his contribution to figure 1. I apologize to all those colleagues whose work could not be cited directly in the manuscript due to
space constraints. The work from my laboratory that is mentioned here was possible thanks to grants and contributions from the Jerôme Lejeune Foundation, Fundació Catalana Síndrome de Down,
the Catalan Government (2009SGR1313), Spanish Ministry of Education and Sciences (SAF2007-60827, SAF2007-31093-E and SAF2010-16427), EU/FIS (PS09102673), CureFXS, ERARare, Fundación Ramón
Areces, Alicia Koplowtiz, Marató TV3 and the Centre for Biomedical Network Research on Rare Diseases. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Genes and Disease Programme, Centre for
Genomic Regulation (CRG); Universitat Pompeu Fabra (UPF), Mara Dierssen * Universitat Pompeu Fabra (UPF), Mara Dierssen * Centro de Investigación Biomédica en Red de Enfermedades Raras
(CIBERER), Dr. Aiguader 88, Barcelona, E-08003, Spain Mara Dierssen Authors * Mara Dierssen View author publications You can also search for this author inPubMed Google Scholar ETHICS
DECLARATIONS COMPETING INTERESTS The author declares no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION S1 (MOVIE) The Tapping test is part of the Wechsler
Memory Scale-Revised and it is an adaptation of the Corsi-Block Tapping test (Orsini, A. Corsi’s block-tapping test: standardization and concurrent validity with WISC-R for children aged 11
to 16. _Percept. Mot. Skills_ 79, 1547–1554 (1994)). It allows the study of the hippocampus-dependent visual-spatial declarative memory but also reflects attentional capacity, visual
perceptive organization and executive functioning. This test is broadly used to define these specific abilities in different intellectual disability syndromes. Even though more sophisticated
techniques are used now and computer versions of this test are available, the original version still provides very useful information. Briefly, the participants are presented in series with
'_n_' elements. In the absence of any difference between the shape and colour of the cubes, the spatial component is the most relevant. Sequences to be reproduced are randomly
selected through a computer program, taking into account that the different spatial configurations provide a similar level of difficulty. Spatial memory is assessed by analysing the errors
in reproducing the correct sequence of element taps. In general, the best recalled items in the series are the initial and final items (primacy and recency effect). Movie S1 shows a correct
reproduction of the series by a healthy volunteer after the experimenter’s demonstration. The numbers are shown to the experimenter but are not visible for the participant. (MP4 314 kb)
SUPPLEMENTARY INFORMATION S2 (MOVIE) Movie S2 shows the same series demonstrated by the experimenter in Movie S1, but here the healthy volunteer is not reproducing the series correctly.
Instead the volunteer is demonstrating a typical visuospatial error, not maintaining the spatial configuration presented. Random errors or inclusion of an element that is not present in the
series are also frequent in populations with intellectual disability. (MP4 364 kb) SUPPLEMENTARY INFORMATION S3 (MOVIE) Neuritogenesis in wild-type mice. The movie shows phase contrast
time-lapse experiments performed to analyse axon growth in EGFP-transfected cultured cortical neurons derived from a 17.5-day-old wild-type mouse embryo (Martinez de Lagran, M. _et al_.
Dyrk1A influences neuronal morphogenesis through regulation of cytoskeletal dynamics in mammalian cortical neurons. _Cereb. Cortex_ 2 Jan 2012 (doi: 10.1093/cercor/bhr362)). Images were
acquired at DIV 1 every 5 min for a period of 14 hours using 40 ms integration time to record stage coordinates of suitable axonal growth cones and analysed with particle track plugging of
Image J software. (AVI 64555 kb) SUPPLEMENTARY INFORMATION S4 (MOVIE) Neuritogenesis in Down syndrome. The movie shows phase contrast time-lapse experiments performed to analyse axon growth
in EGFP-transfected cultured cortical neurons derived from a 17.5-day-old _Dyrk1A_-overexpressing (TgDyrk1A) embryo. Images were acquired as described for Movie S3. Note that the axonal
behaviour is different in transgenic neurons, which showed a significant reduction in the distance travelled by the axon leading to reduced axonal elongation, a phenotype that is also
detected in Down syndrome (for detailed explanation, see Martinez de Lagran, M. _et al_. Dyrk1A influences neuronal morphogenesis through regulation of cytoskeletal dynamics in mammalian
cortical neurons. _Cereb. Cortex_ 2 Jan 2012 (doi: 10.1093/cercor/bhr362)). TgDyrk1A neurons presented with shorter terminal segments and less complex dendritic arbors with fewer dendrites,
branch points and terminal segments. Mature synapse formation is reduced in the TgDyrk1A mouse, where filopodia-like spines are more abundant and mature spines are reduced in number
(Martinez de Lagran, M. _et al_. Dyrk1A influences neuronal morphogenesis through regulation of cytoskeletal dynamics in mammalian cortical neurons. _Cereb. Cortex_ 2 Jan 2012 (doi:
10.1093/cercor/bhr362); Popov, V. I., Kleschevnikov, A. M., Klimenko, O. A., Stewart, M. G. & Belichenko, P. V. Three-dimensional synaptic ultrastructure in the dentate gyrus and
hippocampal area CA3 in the Ts65Dn mouse model of Down syndrome. _J. Comp. Neurol_. 519, 1338–1354 (2011); Tejedor, F. J. & Hammerle, B. MNB/DYRK1A as a multiple regulator of neuronal
development. _FEBS J_. 278, 223–235 (2011); Belichenko, P. V. _et al_. Synaptic structural abnormalities in the Ts65Dn mouse model of Down Syndrome. _J. Comp. Neurol_. 480, 281–298 (2004)).
(AVI 44186 kb) RELATED LINKS RELATED LINKS FURTHER INFORMATION Mara Dierssen's homepage Down Syndrome International: Research and Practice Down Syndrome Research and Treatment
Foundation Gene Function and Pathway Databases — GFuncPathdb The HSA21 expression map initiative: a gene expression map of HSA21 orthologues in the mouse Jerôme Lejeune Foundation The
National Down syndrome Congress Online Mendelian Inheritance in Man: Down syndrome GLOSSARY * Intellectual disability A disability that is characterized by significant limitations both in
intellectual functioning and in adaptive behaviour. * Working memory A system that is involved in the temporary storage and ongoing maintenance of information. * Long-term memory A memory
system for more permanently storing, managing and retrieving information for later use. * Explicit memory This is the conscious processing of information to remember it following a delay. *
Implicit memory This comprises an unconscious, slower learning system, in which a previous experience influences current behaviour without consciousness of the first episode. * Brachycephaly
A condition in which an individual has an abnormally broad and short head, which occurs when the coronal sutures close prematurely. * Plasticity This is defined as the capacity of the
nervous system to modify its structural and functional organization as a result of experience. * Cognition This is considered to be the process or processes whereby an organism gains
knowledge or becomes aware of events or objects in its environment and uses that knowledge for comprehension and problem solving. * Small non-coding RNAs These are regulatory genomic
elements that are 18–30 nucleotides in length and include microRNAs, PIWI-interacting RNAs and endogenous small interfering RNAs. * Cerebral cortex This is the outermost layer of the
cerebral hemispheres of the brain and is largely responsible for all forms of conscious experience, including perception, emotion, thought and planning. RIGHTS AND PERMISSIONS Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Dierssen, M. Down syndrome: the brain in trisomic mode. _Nat Rev Neurosci_ 13, 844–858 (2012). https://doi.org/10.1038/nrn3314 Download
citation * Published: 20 November 2012 * Issue Date: December 2012 * DOI: https://doi.org/10.1038/nrn3314 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative