- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
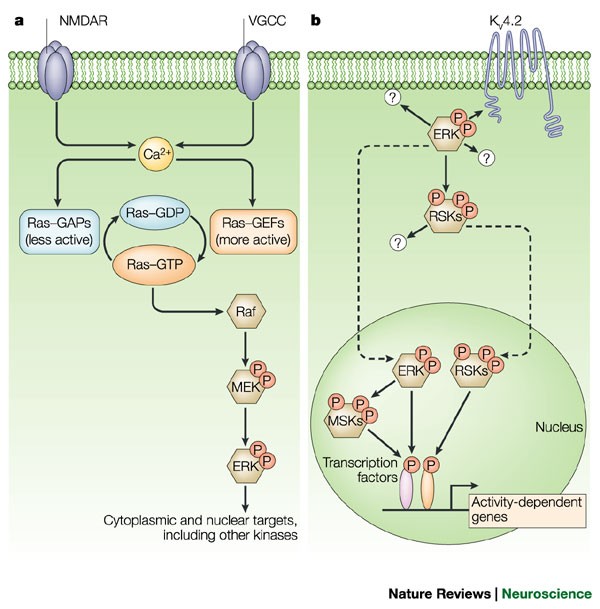
In mature neurons, the mitogen-activated protein kinase (MAPK) cascade that leads to the activation of extracellular signal-regulated kinases (ERKs) is stimulated by excitatory glutamatergic
signalling, and might therefore have a role in synaptic plasticity.
Elucidation of the roles of ERKs in the brain has been aided by the development of specific inhibitors of MAPK cascades. Inhibitors have been used to block the induction of long-term
potentiation in the hippocampus. ERK-dependency has since been demonstrated for many other forms of synaptic plasticity.
Experiments that used inhibitors in behaving animals have indicated that ERK is involved in learning and memory (spatial learning, fear conditioning and conditioned taste aversion).
At the cellular level, ERK activity influences two processes that probably underlie changes in synaptic transmission — the activity of postsynaptic AMPA receptors, and structural plasticity.
Active ERK is present in the nuclei of stimulated cells, as well as in the cytoplasm and dendrites, indicating that the ERK cascade controls phosphorylation of targets both within nuclei and
close to synapses.
Substrates for the ERK cascade during synaptic plasticity probably include the voltage-dependent potassium channel KV4.2, cytoskeletal proteins and transcription factors such as Elk1 and
CREB.
p38 MAPK is highly expressed in the adult brain. Evidence is emerging that the kinase cascade leading to the activation of this MAPK is also a key regulator of synaptic plasticity.
The mitogen-activated protein kinase (MAPK) cascade that leads to the activation of extracellular signal-regulated kinases-1 and -2 (ERK1 and ERK2) has a key role in the differentiation of
some cell types and the proliferation of others. However, several recent reports implicate this cascade in the control of synaptic plasticity in the adult brain. ERK signalling seems to be
essential for characterized neuronal transcriptional events, and might also regulate synaptic targets to control plasticity. Another recently emerging story is the involvement of a
'parallel' but distinct kinase cascade leading to the activation of p38 MAPK, which might control distinct forms of synaptic plasticity.
We thank M. Nuriya and D. Ginty for helpful comments on the manuscript. G.M.T. acknowledges a Wellcome Trust International Prize Travelling Fellowship. Work in the laboratory of R.L.H. is
supported by the Howard Hughes Medical Institute and the National Institutes of Health.
Howard Hughes Medical Institute and Department of Neuroscience, Johns Hopkins University School of Medicine, PCTB 904, 725 North Wolfe Street, Baltimore, 21205, Maryland, USA
(LTP). A possible mechanism for information storage, LTP is a long-lasting strengthening of synaptic responses following specific patterns of 'firing'.
(LTD). An enduring weakening of synaptic strength that is thought to interact with LTP in the cellular mechanisms of learning and memory.
In mammals, depriving one eye of visual input when neuronal connections between the eyes and visual cortex are forming weakens synaptic connections between these areas. Connections from the
'open' eye populate the area of visual cortex that is normally reserved for the deprived eye. The deprived eye becomes functionally disconnected from the visual cortex, leaving the animal
behaviourally blind in this eye, even when visual input is restored.
Elements in the promoter region of many signal-regulated genes, recognized by CREB family transcription factors.
(PSD). In electron micrographs of glutamatergic synapses, an ultrastructural thickening is visible on the postsynaptic side. This PSD can be purified biochemically owing to its resistance to
detergents, and is enriched in NMDA-type glutamate receptors, as well as in many scaffold/adaptor proteins and signalling enzymes.
Anyone you share the following link with will be able to read this content: