- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
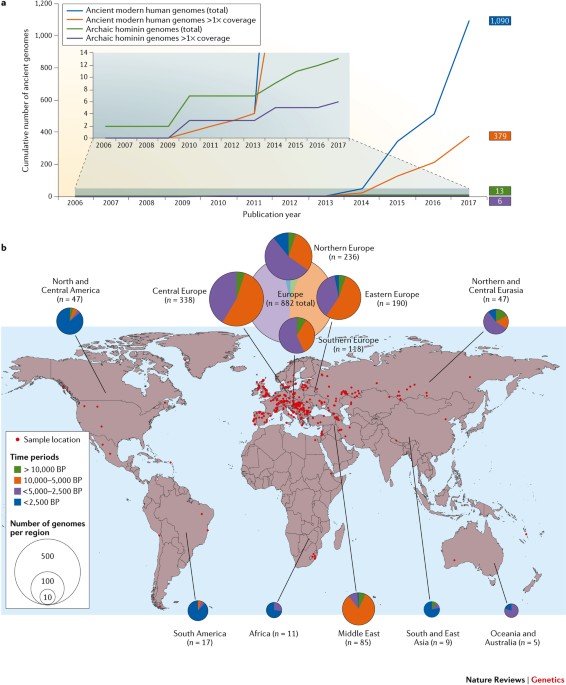
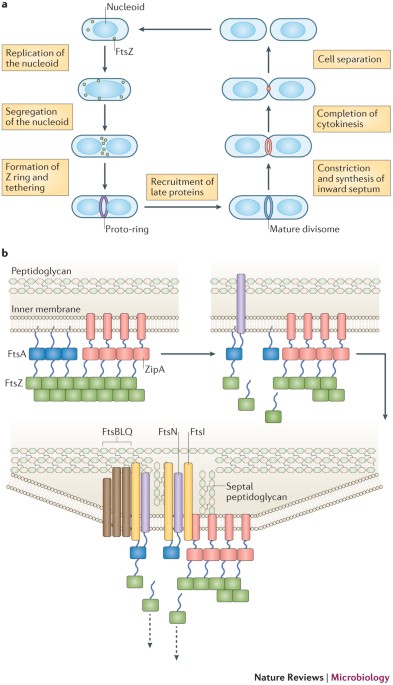
KEY POINTS * All cells must divide to proliferate, and most bacteria divide by splitting themselves into two during cytokinesis. Many bacteria divide by splitting into approximately equal
halves in a process called binary fission. Cytokinesis in bacteria is achieved by the divisome, a dedicated protein machine that is located at the site of cell division. Recent advances in
ultrastructural imaging, biochemistry and genetics of _Escherichia coli_ and other model bacterial species have helped to refine models of divisome function and regulation. * FtsZ, the
bacterial homologue of tubulin, is the principal driver of bacterial cytokinesis. _In vitro_, FtsZ assembles into single protofilaments in the presence of GTP. _In vivo_, these
protofilaments loosely assemble to encircle the cell at the site of division — called the Z ring — and are positioned there by species-specific spatial positioning proteins. * As FtsZ is a
soluble protein, FtsZ protofilaments must be tethered to the inner surface of the cytoplasmic membrane by additional proteins, including FtsA and ZipA in _E. coli_. This complex of FtsZ and
membrane tethers is called the proto-ring and has highly dynamic behaviour. * Although they do not form microtubules, FtsZ protofilaments self-associate to form bundles, either through
interactions with other FtsZ subunits or with several FtsZ-binding proteins that enhance bundling, including ZipA and Zap proteins. These lateral interactions between FtsZ protofilaments may
be important for the ability of FtsZ to divide a cell. * FtsA, a bacterial homologue of actin, is a key connector between the Z ring and other proteins of the divisome, all of which span
the membrane and some of which bind to the peptidoglycan layer. Once the divisome is completely assembled, it coordinates the inward constriction of the Z ring and cytoplasmic membrane with
the synthesis of the cell division septum, which is composed of peptidoglycan. FtsA is a key player in this coordination, which probably involves feedback signalling between the
peptidoglycan-binding divisome proteins and the Z ring. Biochemical characterization of FtsA remains a major challenge. * In addition to signalling in the divisome during the process of
cytokinesis, the divisome is regulated by mechanical, metabolic and stress inputs. FtsZ is a major target for these regulators, but other divisome proteins are also targets. Understanding
how divisome proteins are inhibited or stimulated will be valuable in the future design of divisome-specific antimicrobial compounds. ABSTRACT Bacteria must divide to increase in number and
colonize their niche. Binary fission is the most widespread means of bacterial cell division, but even this relatively simple mechanism has many variations on a theme. In most bacteria, the
tubulin homologue FtsZ assembles into a ring structure, termed the Z ring, at the site of cytokinesis and recruits additional proteins to form a large protein machine — the divisome — that
spans the membrane. In this Review, we discuss current insights into the regulation of the assembly of the Z ring and how the divisome drives membrane invagination and septal cell wall
growth while flexibly responding to various cellular inputs. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS
OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on
SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about
institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS INSIGHTS INTO THE ASSEMBLY AND REGULATION OF THE BACTERIAL DIVISOME Article 31
July 2023 SINGLE-MOLECULE IMAGING REVEALS THAT Z-RING CONDENSATION IS ESSENTIAL FOR CELL DIVISION IN _BACILLUS SUBTILIS_ Article 18 March 2021 FUNCTION AND REGULATION OF THE DIVISOME FOR
MITOCHONDRIAL FISSION Article 03 February 2021 CHANGE HISTORY * _ 25 APRIL 2016 _Nature Reviews Microbiology_ 14, 305–319 (2016). In the sixth paragraph of the section 'FtsA and the
dynamics of proto-ring assembly', the sentence “Finally, an FtsZ mutant with decreased self-bundling _in vitro_ (FtsZ-E93R) has reduced function in cell division50, 51.” should have
read “However, an FtsZ mutant with increased self-bundling _in vitro_ (FtsZ-E93R) has reduced function in cell division50,51.” The authors apologize to the readers for any misunderstanding
caused. _ REFERENCES * Egan, A. J. & Vollmer, W. The physiology of bacterial cell division. _Ann. NY Acad. Sci._ 1277, 8–28 (2013). Article CAS PubMed Google Scholar * Brown, P. J.
et al. Polar growth in the alphaproteobacterial order Rhizobiales. _Proc. Natl Acad. Sci. USA_ 109, 1697–1701 (2011). Article Google Scholar * Margolin, W. Themes and variations in
prokaryotic cell division. _FEMS Microbiol. Rev._ 24, 531–548 (2000). Article CAS PubMed Google Scholar * Leisch, N. et al. Growth in width and FtsZ ring longitudinal positioning in a
gammaproteobacterial symbiont. _Curr. Biol._ 22, R831–R832 (2012). Article CAS PubMed Google Scholar * Monahan, L. G., Liew, A. T., Bottomley, A. L. & Harry, E. J. Division site
positioning in bacteria: one size does not fit all. _Front. Microbiol._ 5, 19 (2014). Article PubMed PubMed Central Google Scholar * Rowlett, V. W. & Margolin, W. The Min system and
other nucleoid-independent regulators of Z ring positioning. _Front. Microbiol._ 6, 478 (2015). Article PubMed PubMed Central Google Scholar * Willemse, J., Borst, J. W., de Waal, E.,
Bisseling, T. & van Wezel, G. P. Positive control of cell division: FtsZ is recruited by SsgB during sporulation of _Streptomyces_. _Genes Dev._ 25, 89–99 (2011).THIS STUDY PROVIDES THE
FIRST EXAMPLE OF POSITIVE SPATIAL REGULATION OF Z RING POSITIONING. Article CAS PubMed PubMed Central Google Scholar * Treuner-Lange, A. et al. PomZ, a ParA-like protein, regulates
Z-ring formation and cell division in _Myxococcus xanthus_. _Mol. Microbiol._ 87, 235–253 (2013). Article CAS PubMed Google Scholar * Holeckova, N. et al. LocZ is a new cell division
protein involved in proper septum placement in _Streptococcus Pneumoniae_. _mBio_ 6, e01700-14 (2014). Article PubMed PubMed Central CAS Google Scholar * Fleurie, A. et al. MapZ marks
the division sites and positions FtsZ rings in _Streptococcus pneumoniae_. _Nature_ 516, 259–262 (2014). Article CAS PubMed PubMed Central Google Scholar * McCormick, J. R., Su, E. P.,
Driks, A. & Losick, R. Growth and viability of _Streptomyces coelicolor_ mutant for the cell division gene _ftsZ_. _Mol. Microbiol._ 14, 243–254 (1994). Article CAS PubMed Google
Scholar * Rico, A. I., Krupka, M. & Vicente, M. In the beginning. _Escherichia coli_ assembled the proto-ring: an initial phase of division. _J. Biol. Chem._ 288, 20830–20836 (2013).
Article CAS PubMed PubMed Central Google Scholar * Pichoff, S. & Lutkenhaus, J. Tethering the Z ring to the membrane through a conserved membrane targeting sequence in FtsA. _Mol.
Microbiol._ 55, 1722–1734 (2005). Article CAS PubMed Google Scholar * Hale, C. A. & de Boer, P. A. Direct binding of FtsZ to ZipA, an essential component of the septal ring structure
that mediates cell division in _E. coli_. _Cell_ 88, 175–185 (1997). Article CAS PubMed Google Scholar * Pichoff, S. & Lutkenhaus, J. Unique and overlapping roles for ZipA and FtsA
in septal ring assembly in _Escherichia coli_. _EMBO J._ 21, 685–693 (2002). Article CAS PubMed PubMed Central Google Scholar * Duman, R. et al. Structural and genetic analyses reveal
the protein SepF as a new membrane anchor for the Z ring. _Proc. Natl Acad. Sci. USA_ 110, E4601–E4610 (2013). Article CAS PubMed PubMed Central Google Scholar * Krol, E. et al.
_Bacillus subtilis_ SepF binds to the C-terminus of FtsZ. _PLoS ONE_ 7, e43293 (2013). Google Scholar * Ishikawa, S., Kawai, Y., Hiramatsu, K., Kuwano, M. & Ogasawara, N. A new
FtsZ-interacting protein, YlmF, complements the activity of FtsA during progression of cell division in _Bacillus subtilis_. _Mol. Microbiol._ 60, 1364–1380 (2006). Article CAS PubMed
Google Scholar * Gupta, S. et al. The essential protein SepF of mycobacteria interacts with FtsZ and MurG to regulate cell growth and division. _Microbiology_ 161, 1627–1638 (2015). Article
CAS PubMed Google Scholar * Gola, S., Munder, T., Casonato, S., Manganelli, R. & Vicente, M. The essential role of SepF in mycobacterial division. _Mol. Microbiol._ 97, 560–576
(2015). Article CAS PubMed Google Scholar * Levin, P. A., Kurtser, I. G. & Grossman, A. D. Identification and characterization of a negative regulator of FtsZ ring formation in
_Bacillus subtilis_. _Proc. Natl Acad. Sci. USA_ 96, 9642–9647 (1999). Article CAS PubMed PubMed Central Google Scholar * Steele, V. R., Bottomley, A. L., Garcia-Lara, J.,
Kasturiarachchi, J. & Foster, S. J. Multiple essential roles for EzrA in cell division of _Staphylococcus aureus_. _Mol. Microbiol._ 80, 542–555 (2011). Article CAS PubMed Google
Scholar * Haeusser, D. P. et al. EzrA prevents aberrant cell division by modulating assembly of the cytoskeletal protein FtsZ. _Mol. Microbiol._ 52, 801–814 (2004). Article CAS PubMed
PubMed Central Google Scholar * Cleverley, R. M. et al. Structure and function of a spectrin-like regulator of bacterial cytokinesis. _Nat. Commun._ 5, 5421 (2014). Article PubMed Google
Scholar * Machnicka, B. et al. Spectrins: a structural platform for stabilization and activation of membrane channels, receptors and transporters. _Biochim. Biophys. Acta_ 1838, 620–634
(2014). Article CAS PubMed Google Scholar * Haeusser, D. P., Garza, A. C., Buscher, A. Z. & Levin, P. A. The division inhibitor EzrA contains a seven-residue patch required for
maintaining the dynamic nature of the medial FtsZ ring. _J. Bacteriol._ 189, 9001–9010 (2007). Article CAS PubMed PubMed Central Google Scholar * Land, A. D., Luo, Q. & Levin, P. A.
Functional domain analysis of the cell division inhibitor EzrA. _PLoS ONE_ 9, e102616 (2014). Article PubMed PubMed Central CAS Google Scholar * Son, S. H. & Lee, H. H. The
N-terminal domain of EzrA binds to the C terminus of FtsZ to inhibit _Staphylococcus aureus_ FtsZ polymerization. _Biochem. Biophys. Res. Commun._ 433, 108–114 (2013). Article CAS PubMed
Google Scholar * van den Ent, F. & Löwe, J. Crystal structure of the cell division protein FtsA from _Thermotoga maritima_. _EMBO J._ 19, 5300–5307 (2000). Article CAS PubMed PubMed
Central Google Scholar * Sanchez, M., Valencia, A., Ferrandiz, M. J., Sandler, C. & Vicente, M. Correlation between the structure and biochemical activities of FtsA, an essential cell
division protein of the actin family. _EMBO J._ 13, 4919–4925 (1994). Article CAS PubMed PubMed Central Google Scholar * Feucht, A., Lucet, I., Yudkin, M. D. & Errington, J.
Cytological and biochemical characterization of the FtsA cell division protein of _Bacillus subtilis_. _Mol. Microbiol._ 40, 115–125 (2001). Article CAS PubMed Google Scholar *
Paradis-Bleau, C., Sanschagrin, F. & Levesque, R. C. Peptide inhibitors of the essential cell division protein FtsA. _Protein Eng. Des. Sel._ 18, 85–91 (2005). Article CAS PubMed
Google Scholar * Lara, B. et al. Cell division in cocci: localization and properties of the _Streptococcus pneumoniae_ FtsA protein. _Mol. Microbiol._ 55, 699–711 (2005).THE FIRST STUDY TO
DEMONSTRATE THAT FTSA HAS ATP-DEPENDENT POLYMERIZATION ACTIVITY. Article CAS PubMed Google Scholar * Szwedziak, P., Wang, Q., Freund, S. M. & Löwe, J. FtsA forms actin-like
protofilaments. _EMBO J._ 31, 2249–2260 (2012).THIS STUDY STRUCTURALLY DEFINES THE FTSA–FTSA AND FTSA–FTSZ INTERFACES. Article CAS PubMed PubMed Central Google Scholar * Pichoff, S.,
Shen, B., Sullivan, B. & Lutkenhaus, J. FtsA mutants impaired for self-interaction bypass ZipA suggesting a model in which FtsA's self-interaction competes with its ability to
recruit downstream division proteins. _Mol. Microbiol._ 83, 151–167 (2012).THIS STUDY SHOWS THAT MANY MUTATIONS THAT INTERFERE WITH FTSA OLIGOMERIZATION RENDER ZIPA NON-ESSENTIAL. Article
CAS PubMed Google Scholar * Shiomi, D. & Margolin, W. Dimerization or oligomerization of the actin-like FtsA protein enhances the integrity of the cytokinetic Z ring. _Mol.
Microbiol._ 66, 1396–1415 (2007). CAS PubMed PubMed Central Google Scholar * Fujita, J. et al. Crystal structure of FtsA from _Staphylococcus aureus_. _FEBS Lett._ 588, 1879–1885 (2014).
Article CAS PubMed Google Scholar * Geissler, B., Elraheb, D. & Margolin, W. A gain of function mutation in _ftsA_ bypasses the requirement for the essential cell division gene
_zipA_ in _Escherichia coli_. _Proc. Natl Acad. Sci. USA_ 100, 4197–4202 (2003). Article CAS PubMed PubMed Central Google Scholar * Geissler, B. & Margolin, W. Evidence for
functional overlap among multiple bacterial cell division proteins: compensating for the loss of FtsK. _Mol. Microbiol._ 58, 596–612 (2005). Article CAS PubMed PubMed Central Google
Scholar * Beuria, T. K. et al. Adenine nucleotide-dependent regulation of assembly of bacterial tubulin-like FtsZ by a hypermorph of bacterial actin-like FtsA. _J. Biol. Chem._ 284,
14079–14086 (2009).THE FIRST EVIDENCE THAT FTSA CAN AFFECT THE ASSEMBLY STATE OF FTSZ. Article CAS PubMed PubMed Central Google Scholar * Loose, M. & Mitchison, T. J. The bacterial
cell division proteins FtsA and FtsZ self-organize into dynamic cytoskeletal patterns. _Nat. Cell Biol._ 16, 38–46 (2014).THIS STUDY SHOWS THAT FTSA CAN REGULATE FTSZ ASSEMBLY DYNAMICS ON A
PHYSIOLOGICAL MEMBRANE SURFACE. Article CAS PubMed Google Scholar * Modi, K. & Misra, H. S. Dr-FtsA, an actin homologue in _Deinococcus radiodurans_ differentially affects Dr-FtsZ
and Ec-FtsZ functions _in vitro_. _PLoS ONE_ 9, e115918 (2014). Article PubMed PubMed Central CAS Google Scholar * Du, S., Park, K.-T. & Lutkenhaus, J. Oligomerization of FtsZ
converts the FtsZ tail motif (CCTP) into a multivalent ligand with high avidity for partners ZipA and SlmA. _Mol. Microbiol._ 95, 173–188 (2015). Article CAS PubMed Google Scholar *
Herricks, J. R., Nguyen, D. & Margolin, W. A thermosensitive defect in the ATP binding pocket of FtsA can be suppressed by allosteric changes in the dimer interface. _Mol. Microbiol._
94, 713–727 (2014). Article CAS PubMed PubMed Central Google Scholar * Shen, B. & Lutkenhaus, J. The conserved C-terminal tail of FtsZ is required for the septal localization and
division inhibitory activity of MinCC/MinD. _Mol. Microbiol._ 72, 410–424 (2009). Article CAS PubMed PubMed Central Google Scholar * Rowlett, V. W. & Margolin, W. Asymmetric
constriction of dividing _Escherichia coli_ cells induced by expression of a fusion between two Min proteins. _J. Bacteriol._ 196, 2089–2100 (2014). Article PubMed PubMed Central CAS
Google Scholar * Szwedziak, P., Wang, Q., Bharat, T. A. M., Tsim, M. & Löwe, J. Architecture of the ring formed by the tubulin homologue FtsZ in bacterial cell division. _eLife_ 3,
e04601 (2014). Article PubMed PubMed Central Google Scholar * Hernandez-Rocamora, V. M. et al. Dynamic interaction of the _Escherichia coli_ cell division ZipA and FtsZ proteins
evidenced in nanodiscs. _J. Biol. Chem._ 287, 30097–30104 (2012). Article CAS PubMed PubMed Central Google Scholar * Skoog, K. & Daley, D. O. The _Escherichia coli_ cell division
protein ZipA forms homodimers prior to association with FtsZ. _Biochemistry_ 51, 1407–1415 (2012). Article CAS PubMed Google Scholar * Jaiswal, R. et al. E93R substitution of
_Escherichia coli_ FtsZ induces bundling of protofilaments, reduces GTPase activity, and impairs bacterial cytokinesis. _J. Biol. Chem._ 285, 31796–31805 (2010). Article CAS PubMed PubMed
Central Google Scholar * Haeusser, D. P., Rowlett, V. W. & Margolin, W. A mutation in _Escherichia coli ftsZ_ bypasses the requirement for the essential division gene _zipA_ and
confers resistance to FtsZ assembly inhibitors by stabilizing protofilament bundling. _Mol. Microbiol._ 97, 988–1005 (2015). Article CAS PubMed PubMed Central Google Scholar * Dewar, S.
J., Begg, K. J. & Donachie, W. D. Inhibition of cell division initiation by an imbalance in the ratio of FtsA to FtsZ. _J. Bacteriol._ 174, 6314–6316 (1992). Article CAS PubMed
PubMed Central Google Scholar * Mukherjee, A. & Lutkenhaus, J. Dynamic assembly of FtsZ regulated by GTP hydrolysis. _EMBO J._ 17, 462–469 (1998). Article CAS PubMed PubMed Central
Google Scholar * Scheffers, D. J., de Wit, J. G., den Blaauwen, T. & Driessen, A. J. GTP hydrolysis of cell division protein FtsZ: evidence that the active site is formed by the
association of monomers. _Biochemistry_ 41, 521–529 (2002). Article CAS PubMed Google Scholar * Huang, K. H., Durand-Heredia, J. & Janakiraman, A. FtsZ ring stability: of bundles,
tubules, crosslinks, and curves. _J. Bacteriol._ 195, 1859–1868 (2013). Article CAS PubMed PubMed Central Google Scholar * Meier, E. L. & Goley, E. D. Form and function of the
bacterial cytokinetic ring. _Curr. Opin. Cell Biol._ 26, 147 (2014). Article CAS Google Scholar * Rowlett, V. W. & Margolin, W. The bacterial divisome: ready for its close-up. _Phil.
Trans. R. Soc. Lond. B Biol. Sci._ 370, 20150028 (2015). Article CAS Google Scholar * Li, Z., Trimble, M. J., Brun, Y. V. & Jensen, G. J. The structure of FtsZ filaments _in vivo_
suggests a force-generating role in cell division. _EMBO J._ 26, 4694–4708 (2007).THIS STUDY PROVIDES A GLIMPSE OF THE Z RING AT HIGH RESOLUTION BY ELECTRON TOMOGRAPHY. Article CAS PubMed
PubMed Central Google Scholar * Holden, S. J. et al. High throughput 3D super-resolution microscopy reveals _Caulobacter crescentus in vivo_ Z-ring organization. _Proc. Natl Acad. Sci.
USA_ 111, 4566–4571 (2014). Article CAS PubMed PubMed Central Google Scholar * Jacq, M. et al. Remodeling of the Z-ring nanostructure during the _Streptococcus pneumoniae_ cell cycle
revealed by photoactivated localization microscopy. _mBio_ 6, e01108-15 (2015). Article PubMed PubMed Central CAS Google Scholar * Strauss, M. P. et al. 3D-SIM super resolution
microscopy reveals a bead-like arrangement for FtsZ and the division machinery: implications for triggering cytokinesis. _PLoS Biol._ 10, e1001389 (2012).THIS PAPER PROVIDES THE DIRECT
VISUAL EVIDENCE THAT THE Z RING MAY BE A NON-UNIFORM STRUCTURE. Article CAS PubMed PubMed Central Google Scholar * Rowlett, V. W. & Margolin, W. 3D-SIM super-resolution of FtsZ and
its membrane tethers in _Escherichia coli_ cells. _Biophys. J._ 107, L17–L20 (2014). Article CAS PubMed PubMed Central Google Scholar * Tsui, H. C. T. et al. Pbp2x localizes separately
from Pbp2b and other peptidoglycan synthesis proteins during later stages of cell division of _Streptococcus pneumoniae_ D39. _Mol. Microbiol._ 94, 21–40 (2014). Article CAS PubMed PubMed
Central Google Scholar * Piro, O., Carmon, G., Feingold, M. & Fishov, I. Three-dimensional structure of the Z-ring as a random network of FtsZ filaments. _Env. Microbiol._ 15,
3252–3258 (2013). Article CAS Google Scholar * Si, F., Busiek, K., Margolin, W. & Sun, S. X. Organization of FtsZ filaments in the bacterial division ring measured from polarized
fluorescence microscopy. _Biophys. J._ 105, 1976–1986 (2013). Article CAS PubMed PubMed Central Google Scholar * Judd, E. M. et al. Distinct constrictive processes, separated in time
and space, divide _Caulobacter_ inner and outer membranes. _J. Bacteriol._ 187, 6874–6882 (2005). Article CAS PubMed PubMed Central Google Scholar * Gundogdu, M. E. et al. Large ring
polymers align FtsZ polymers for normal septum formation. _EMBO J._ 30, 617–626 (2011). Article PubMed PubMed Central CAS Google Scholar * Hale, C. A., Rhee, A. C. & de Boer, P. A.
ZipA-induced bundling of FtsZ polymers mediated by an interaction between C-terminal domains. _J. Bacteriol._ 182, 5153–5166 (2000). Article CAS PubMed PubMed Central Google Scholar *
Roach, E. J., Kimber, M. S. & Khursigara, C. M. Crystal structure and site-directed mutational analysis reveals key residues involved in _Escherichia coli_ ZapA function. _J. Biol.
Chem._ 289, 23276–23286 (2014). Article CAS PubMed PubMed Central Google Scholar * Durand-Heredia, J. M., Yu, H. H., De Carlo, S., Lesser, C. F. & Janakiraman, A. Identification and
characterization of ZapC, a stabilizer of the FtsZ ring in _Escherichia coli_. _J. Bacteriol._ 193, 1405–1413 (2011). Article CAS PubMed PubMed Central Google Scholar * Hale, C. A. et
al. Identification of _Escherichia coli_ ZapC (YcbW) as a component of the division apparatus that binds and bundles FtsZ polymers. _J. Bacteriol._ 193, 1393–1404 (2011). Article CAS
PubMed PubMed Central Google Scholar * Durand-Heredia, J., Rivkin, E., Fan, G., Morales, J. & Janakiraman, A. Identification of ZapD as a cell division factor that promotes the
assembly of FtsZ in _Escherichia coli_. _J. Bacteriol._ 194, 3189–3198 (2012). Article CAS PubMed PubMed Central Google Scholar * Buss, J. et al. _In vivo_ organization of the FtsZ-ring
by ZapA and ZapB revealed by quantitative super-resolution microscopy. _Mol. Microbiol._ 89, 1099–1120 (2013). Article CAS PubMed PubMed Central Google Scholar * Gardner, K. A., Moore,
D. A. & Erickson, H. P. The C-terminal linker of _Escherichia coli_ FtsZ functions as an intrinsically disordered peptide. _Mol. Microbiol._ 89, 264–275 (2013). Article CAS PubMed
PubMed Central Google Scholar * Buske, P. J. & Levin, P. A. A flexible C-terminal linker is required for proper FtsZ assembly _in vitro_ and cytokinetic ring formation _in vivo_. _Mol.
Microbiol._ 89, 249–263 (2013). Article CAS PubMed PubMed Central Google Scholar * Sundararajan, K. et al. The bacterial tubulin FtsZ requires its intrinsically disordered linker to
direct robust cell wall construction. _Nat. Commun._ 6, 7281 (2015). Article CAS PubMed Google Scholar * Buske, P. J. & Levin, P. A. Extreme C-terminus of bacterial cytoskeletal
protein FtsZ plays fundamental role in assembly independent of modulatory proteins. _J. Biol. Chem._ 287, 10945–10957 (2012). Article CAS PubMed PubMed Central Google Scholar * Fu, G.
et al. _in vivo_ structure of the _E. coli_ FtsZ-ring revealed by photoactivated localization microscopy (PALM). _PLoS ONE_ 5, e12682 (2010). Article PubMed CAS Google Scholar * Aarsman,
M. E. et al. Maturation of the _Escherichia coli_ divisome occurs in two steps. _Mol. Microbiol._ 55, 1631–1645 (2005). Article CAS PubMed Google Scholar * Gamba, P., Veening, J. W.,
Saunders, N. J., Hamoen, L. W. & Daniel, R. A. Two-step assembly dynamics of the _Bacillus subtilis_ divisome. _J. Bacteriol._ 191, 4186–4194 (2009). Article CAS PubMed PubMed Central
Google Scholar * Mohammadi, T. et al. Identification of FtsW as a transporter of lipid-linked cell wall precursors across the membrane. _EMBO J._ 30, 1425–1432 (2011). Article CAS
PubMed PubMed Central Google Scholar * Sham, L. T. et al. Bacterial cell wall. MurJ is the flippase of lipid-linked precursors for peptidoglycan biogenesis. _Science_ 345, 220–222 (2014).
Article CAS PubMed PubMed Central Google Scholar * Van den Berg van Saparoea, H. B. et al. Fine-mapping the contact sites of the _Escherichia coli_ cell division proteins FtsB and FtsL
on the FtsQ protein. _J. Biol. Chem._ 288, 24340–24350 (2013). Article CAS PubMed PubMed Central Google Scholar * Khadria, A. S. & Senes, A. The transmembrane domains of the
bacterial cell division proteins FtsB and FtsL form a stable high-order oligomer. _Biochemistry_ 52, 7542–7550 (2013). Article CAS PubMed Google Scholar * Glas, M. et al. The soluble
periplasmic domains of _E. coli_ cell division proteins FtsQ/FtsB/FtsL form a trimeric complex with sub-micromolar affinity. _J. Biol. Chem._ 290, 21498–21509 (2015). Article CAS PubMed
PubMed Central Google Scholar * Bottomley, A. L. et al. _Staphylococcus aureus_ DivIB is a peptidoglycan-binding protein that is required for a morphological checkpoint in cell division.
_Mol. Microbiol._ 94, 1041–1064 (2014). Article CAS Google Scholar * Grenga, L., Rizzo, A., Paolozzi, L. & Ghelardini, P. Essential and non-essential interactions in interactome
networks: the _Escherichia coli_ division proteins FtsQ–FtsN interaction. _Env. Microbiol._ 15, 3210–3217 (2013). Article CAS Google Scholar * Alexeeva, S. et al. Direct interactions of
early and late assembling division proteins in _Escherichia coli_ cells resolved by FRET. _Mol. Microbiol._ 77, 384–398 (2010). Article CAS PubMed Google Scholar * Busiek, K. K., Eraso,
J. M., Wang, Y. & Margolin, W. The early divisome protein FtsA interacts directly through its 1c subdomain with the cytoplasmic domain of the late divisome protein FtsN. _J. Bacteriol._
194, 1989–2000 (2012). Article CAS PubMed PubMed Central Google Scholar * Rico, A. I., Garcia-Ovalle, M., Mingorance, J. & Vicente, M. Role of two essential domains of _Escherichia
coli_ FtsA in localization and progression of the division ring. _Mol. Microbiol._ 53, 1359–1371 (2004). Article CAS PubMed Google Scholar * Yahashiri, A., Jorgenson, M. A. & Weiss,
D. S. Bacterial SPOR domains are recruited to septal peptidoglycan by binding to glycan strands that lack stem peptides. _Proc. Natl Acad. Sci. USA_ 112, 11347–11352 (2015). Article CAS
PubMed PubMed Central Google Scholar * Gerding, M. A. et al. Self-enhanced accumulation of FtsN at division sites and roles for other proteins with a SPOR domain (DamX, DedD, and RlpA) in
_Escherichia coli_ cell constriction. _J. Bacteriol._ 191, 7383–7401 (2009). Article CAS PubMed PubMed Central Google Scholar * Busiek, K. K. & Margolin, W. A role for FtsA in
SPOR-independent localization of the essential _Escherichia coli_ cell division protein FtsN. _Mol. Microbiol._ 92, 1212–1226 (2014). Article CAS PubMed PubMed Central Google Scholar *
Bernard, C. S., Sadasivam, M., Shiomi, D. & Margolin, W. An altered FtsA can compensate for the loss of essential cell division protein FtsN in _Escherichia coli_. _Mol. Microbiol._ 64,
1289–1305 (2007). Article CAS PubMed PubMed Central Google Scholar * Liu, B., Persons, L., Lee, L. & de Boer, P. Roles for both FtsA and the FtsBLQ subcomplex in FtsN-stimulated
cell constriction in _Escherichia coli_. _Mol. Microbiol._ 95, 945–970 (2015).TOGETHER WITH REFERENCES 89, 93 AND 97, THIS STUDY PROVIDES ADDITIONAL EVIDENCE FOR SEVERAL SIGNALLING PATHWAYS
IN THE DIVISOME TO ACTIVATE CYTOKINESIS. Article CAS PubMed PubMed Central Google Scholar * Pichoff, S., Du, S. & Lutkenhaus, J. The bypass of ZipA by overexpression of FtsN
requires a previously unknown conserved FtsN motif essential for FtsA–FtsN interaction supporting a model in which FtsA monomers recruit late cell division proteins to the Z ring. _Mol.
Microbiol._ 95, 971–987 (2015). Article CAS PubMed PubMed Central Google Scholar * Tsang, M. J. & Bernhardt, T. G. A role for the FtsQLB complex in cytokinetic ring activation
revealed by an _ftsL_ allele that accelerates division. _Mol. Microbiol._ 95, 925–944 (2015). Article CAS PubMed PubMed Central Google Scholar * Potluri, L. P., Kannan, S. & Young,
K. D. ZipA is required for FtsZ-dependent preseptal peptidoglycan synthesis prior to invagination during cell division. _J. Bacteriol._ 194, 5334–5342 (2012). Article CAS PubMed PubMed
Central Google Scholar * Claessen, D. et al. Control of the cell elongation–division cycle by shuttling of PBP1 protein in _Bacillus subtilis_. _Mol. Microbiol._ 68, 1029–1046 (2008).
Article CAS PubMed Google Scholar * Land, A. D. et al. Requirement of essential Pbp2x and GpsB for septal ring closure in _Streptococcus pneumoniae_ D39. _Mol. Microbiol._ 90, 939–955
(2013). Article CAS PubMed PubMed Central Google Scholar * Fleurie, A. et al. Interplay of the serine/threonine-kinase StkP and the paralogs DivIVA and GpsB in pneumococcal cell
elongation and division. _PLoS Genet._ 10, e1004275 (2014). Article PubMed PubMed Central CAS Google Scholar * Pompeo, F., Foulquier, E., Serrano, B., Grangeasse, C. & Galinier, A.
Phosphorylation of the cell division protein GpsB regulates PrkC kinase activity through a negative feedback loop in _Bacillus subtilis_. _Mol. Microbiol._ 97, 139–150 (2015). Article CAS
PubMed Google Scholar * Gerding, M. A., Ogata, Y., Pecora, N. D., Niki, H. & de Boer, P. A. The _trans_-envelope Tol–Pal complex is part of the cell division machinery and required for
proper outer-membrane invagination during cell constriction in _E. coli_. _Mol. Microbiol._ 63, 1008–1025 (2007).THE FIRST MECHANISM FOR COORDINATING INNER MEMBRANE AND OUTER MEMBRANE
CONSTRICTION DURING CYTOKINESIS IN A GRAM-NEGATIVE BACTERIUM. Article CAS PubMed PubMed Central Google Scholar * Gray, A. N. et al. Coordination of peptidoglycan synthesis and outer
membrane constriction during _Escherichia coli_ cell division. _eLife_ 4, e07118 (2015). Article PubMed Central Google Scholar * Corbin, B. D., Wang, Y., Beuria, T. K. & Margolin, W.
Interaction between cell division proteins FtsE and FtsZ. _J. Bacteriol._ 189, 3026–3035 (2007). Article CAS PubMed PubMed Central Google Scholar * Yang, D. C. et al. An ATP-binding
cassette transporter-like complex governs cell-wall hydrolysis at the bacterial cytokinetic ring. _Proc. Natl Acad. Sci. USA_ 108, E1052–E1060 (2011). Article PubMed PubMed Central Google
Scholar * Jorgenson, M. A., Chen, Y., Yahashiri, A., Popham, D. L. & Weiss, D. S. The bacterial septal ring protein RlpA is a lytic transglycosylase that contributes to rod shape and
daughter cell separation in _Pseudomonas aeruginosa_. _Mol. Microbiol._ 93, 113–128 (2014). Article CAS PubMed PubMed Central Google Scholar * Osawa, M., Anderson, D. E. & Erickson,
H. P. Reconstitution of contractile FtsZ rings in liposomes. _Science_ 320, 792–794 (2008).THE FIRST STUDY TO DEMONSTRATE THAT Z RINGS CAN BE RECONSTITUTED _IN VITRO_. Article CAS PubMed
PubMed Central Google Scholar * Osawa, M. & Erickson, H. P. Liposome division by a simple bacterial division machinery. _Proc. Natl Acad. Sci. USA_ 110, 11000–11004 (2013).THIS STUDY
SHOWS THAT PURIFIED FTSZ AND FTSA* ARE SUFFICIENT TO CONSTRICT LIPOSOMES. Article CAS PubMed PubMed Central Google Scholar * Dow, C. E., van den Berg, H. A., Roper, D. I. & Rodger,
A. Biological insights from a simulation model of the critical FtsZ accumulation required for prokaryotic cell division. _Biochemistry_ 54, 3803–3813 (2015). Article CAS PubMed Google
Scholar * Li, Y. et al. FtsZ protofilaments use a hinge-opening mechanism for constrictive force generation. _Science_ 341, 392–395 (2013).THIS PAPER PROPOSES A MECHANISM FOR FORCE
GENERATION BY FTSZ FILAMENTS ATTACHED TO MEMBRANES. Article CAS PubMed Google Scholar * Söderström, B. et al. Disassembly of the divisome in _Escherichia coli_: evidence that FtsZ
dissociates before compartmentalization. _Mol. Microbiol._ 92, 1–9 (2014). Article PubMed PubMed Central CAS Google Scholar * Krupka, M. et al. Role of the FtsA C-terminus as a switch
for polymerization and membrane association. _mBio_ 5, e02221 (2014).THIS STUDY FURTHER DEFINES THE ROLE OF ATP BINDING FOR FTSA ACTIVITY. Article CAS PubMed PubMed Central Google
Scholar * Cabre, E. J. et al. Bacterial division proteins FtsZ and ZipA induce vesicle shrinkage and cell membrane invagination. _J. Biol. Chem._ 288, 26625–26634 (2013). Article CAS
PubMed PubMed Central Google Scholar * Weiss, D. S. Last but not least: new insights into how FtsN triggers constriction during _Escherichia coli_ cell division. _Mol. Microbiol._ 903–909
(2015). Article CAS PubMed Google Scholar * Tsang, M. J. & Bernhardt, T. G. Guiding divisome assembly and controlling its activity. _Curr. Opin. Microbiol._ 24, 60–65 (2015).
Article CAS PubMed PubMed Central Google Scholar * Weart, R. B. et al. A metabolic sensor governing cell size in bacteria. _Cell_ 130, 335–347 (2007).THIS STUDY IS THE FIRST TO
DEMONSTRATE A MECHANISM FOR METABOLIC REGULATION OF BACTERIAL CELL DIVISION. Article CAS PubMed PubMed Central Google Scholar * Chien, A. C., Zareh, S. K., Wang, Y. M. & Levin, P.
A. Changes in the oligomerization potential of the division inhibitor UgtP co-ordinate _Bacillus subtilis_ cell size with nutrient availability. _Mol. Microbiol._ 86, 594–610 (2012). Article
CAS PubMed PubMed Central Google Scholar * Hill, N. S., Buske, P. J., Shi, Y. & Levin, P. A. A moonlighting enzyme links _Escherichia coli_ cell size with central metabolism. _PLoS
Genet._ 9, e1003663 (2013). Article CAS PubMed PubMed Central Google Scholar * Surdova, K. et al. The conserved DNA-binding protein WhiA is involved in cell division in _Bacillus
subtilis_. _J. Bacteriol._ 195, 5450–5460 (2013). Article CAS PubMed PubMed Central Google Scholar * Monahan, L. G., Hajduk, I. V., Blaber, S. P., Charles, I. G. & Harry, E. J.
Coordinating bacterial cell division with nutrient availability: a role for glycolysis. _mBio_ 5, e00935-14 (2014). Article PubMed PubMed Central CAS Google Scholar * Radhakrishnan, S.
K., Pritchard, S. & Viollier, P. H. Coupling prokaryotic cell fate and division control with a bifunctional and oscillating oxidoreductase homolog. _Dev. Cell_ 18, 90–101 (2010). Article
CAS PubMed Google Scholar * Beaufay, F. et al. A NAD-dependent glutamate dehydrogenase coordinates metabolism with cell division in _Caulobacter crescentus_. _EMBO J._ 34, 1786–1800
(2015). Article CAS PubMed PubMed Central Google Scholar * Takada, H. et al. An essential enzyme for phospholipid synthesis associates with the _Bacillus subtilis_ divisome. _Mol.
Microbiol._ 91, 242–255 (2014). Article CAS PubMed Google Scholar * Weart, R. B., Nakano, S., Lane, B. E., Zuber, P. & Levin, P. A. The ClpX chaperone modulates assembly of the
tubulin-like protein FtsZ. _Mol. Microbiol._ 57, 238–249 (2005). Article CAS PubMed PubMed Central Google Scholar * Camberg, J. L., Hoskins, J. R. & Wickner, S. The interplay of
ClpXP with the cell division machinery in _Escherichia coli_. _J. Bacteriol._ 193, 1911–1918 (2011). Article CAS PubMed PubMed Central Google Scholar * Williams, B., Bhat, N., Chien, P.
& Shapiro, L. ClpXP and ClpAP proteolytic activity on divisome substrates is differentially regulated following the _Caulobacter_ asymmetric cell division. _Mol. Microbiol._ 93, 853–866
(2014). Article CAS PubMed PubMed Central Google Scholar * Chen, Y., Milam, S. L. & Erickson, H. P. SulA inhibits assembly of FtsZ by a simple sequestration mechanism.
_Biochemistry_ 51, 3100–3109 (2012). Article CAS PubMed Google Scholar * Modell, J. W., Hopkins, A. C. & Laub, M. T. DNA damage checkpoint in _Caulobacter crescentus_ inhibits cell
division through a direct interaction with FtsW. _Genes Dev._ 25, 1328–1343 (2011). Article CAS PubMed PubMed Central Google Scholar * Modell, J. W., Kambara, T. K., Perchuk, B. S.
& Laub, M. T. DNA damage-induced, SOS-independent checkpoint regulates cell division in _Caulobacter crescentus_. _PLoS Biol._ 12, e1001977 (2014). Article PubMed PubMed Central CAS
Google Scholar * Mo, A. H. & Burkholder, W. F. YneA, an SOS-induced inhibitor of cell division in _Bacillus subtilis_, is regulated posttranslationally and requires the transmembrane
region for activity. _J. Bacteriol._ 192, 3159–3173 (2010). Article CAS PubMed PubMed Central Google Scholar * Buchholz, M., Nahrstedt, H., Pillukat, M. H., Deppe, V. & Meinhardt,
F. _yneA_ mRNA instability is involved in temporary inhibition of cell division during the SOS response of _Bacillus megaterium_. _Microbiology_ 159, 1564–1574 (2013). Article CAS PubMed
Google Scholar * Marteyn, B. S. et al. ZapE is a novel cell division protein interacting with FtsZ and modulating the Z-ring dynamics. _mBio_ 5, e00022-14 (2014). Article PubMed PubMed
Central CAS Google Scholar * Koprowski, P. et al. Cytoplasmic domain of MscS interacts with cell division protein FtsZ: A possible non-channel function of the mechanosensitive channel in
_Escherichia coli_. _PLoS ONE_ 10, e0127029 (2015). Article PubMed PubMed Central CAS Google Scholar * Wilson, M. E., Jensen, G. S. & Haswell, E. S. Two mechanosensitive channel
homologs influence division ring placement in _Arabidopsis_ chloroplasts. _Plant Cell_ 23, 2939–2949 (2011). Article CAS PubMed PubMed Central Google Scholar * Mercier, R., Kawai, Y.
& Errington, J. General principles for the formation and proliferation of a wall-free (L-form) state in bacteria. _eLife_ 3, e04629 (2014). Article PubMed Central Google Scholar *
Lock, R. L. & Harry, E. J. Cell-division inhibitors: new insights for future antibiotics. _Nat. Rev. Drug Discov._ 7, 324–338 (2008). Article CAS PubMed Google Scholar * Ojima, I.,
Kumar, K., Awasthi, D. & Vineberg, J. G. Drug discovery targeting cell division proteins, microtubules and FtsZ. _Bioorg. Med. Chem._ 22, 5060–5077 (2014). Article CAS PubMed PubMed
Central Google Scholar * Sass, P. & Brotz-Oesterhelt, H. Bacterial cell division as a target for new antibiotics. _Curr. Opin. Microbiol._ 16, 522–530 (2013). Article CAS PubMed
Google Scholar * Artola, M. et al. Effective GTP-replacing FtsZ inhibitors and antibacterial mechanism of action. _ACS Chem. Biol._ 10, 834–843 (2014). Article PubMed CAS Google Scholar
* Haeusser, D. P. et al. The Kil peptide of bacteriophage λ blocks _Escherichia coli_ cytokinesis via ZipA-dependent inhibition of FtsZ assembly. _PLoS Genet._ 10, e1004217 (2014). Article
PubMed PubMed Central CAS Google Scholar * Kiro, R. et al. Gene product 0.4 increases bacteriophage T7 competitiveness by inhibiting host cell division. _Proc. Natl Acad. Sci. USA_
110, 19549–19554 (2013). Article CAS PubMed PubMed Central Google Scholar * Hernandez-Rocamora, V. M., Alfonso, C., Margolin, W., Zorrilla, S. & Rivas, G. Evidence that
bacteriophage λ Kil peptide inhibits bacterial cell division by disrupting FtsZ protofilaments and sequestering protein subunits. _J. Biol. Chem._ 290, 20325–20335 (2015). Article CAS
PubMed PubMed Central Google Scholar * Ballesteros-Plaza, D., Holguera, I., Scheffers, D. J., Salas, M. & Munoz-Espin, D. Phage ϕ29 protein p1 promotes replication by associating with
the FtsZ ring of the divisome in _Bacillus subtilis_. _Proc. Natl Acad. Sci. USA_ 110, 12313–12318 (2013). Article CAS PubMed PubMed Central Google Scholar * Bisson-Filho, A. W. et al.
FtsZ filament capping by MciZ, a developmental regulator of bacterial division. _Proc. Natl Acad. Sci. USA_ 112, E2130–E2138 (2015). Article CAS PubMed PubMed Central Google Scholar *
Pende, N. et al. Size-independent symmetric division in extraordinarily long cells. _Nat. Commun._ 5, 4803 (2014). Article CAS PubMed Google Scholar * Donovan, C. & Bramkamp, M. Cell
division in Corynebacterineae. _Front. Microbiol._ 5, 132 (2014). Article PubMed PubMed Central Google Scholar * Kieser, K. J. & Rubin, E. J. How sisters grow apart: mycobacterial
growth and division. _Nat. Rev. Microbiol._ 12, 550–562 (2014). Article CAS PubMed PubMed Central Google Scholar * Ramos-Leon, F., Mariscal, V., Frias, J. E., Flores, E. & Herrero,
A. Divisome-dependent subcellular localization of cell-cell joining protein SepJ in the filamentous cyanobacterium _Anabaena_. _Mol. Microbiol._ 96, 566–580 (2015). Article CAS PubMed
Google Scholar * Pinho, M. G., Kjos, M. & Veening, J.-W. How to get (a)round: mechanisms controlling growth and division of coccoid bacteria. _Nat. Rev. Microbiol._ 11, 601–614 (2013).
Article CAS PubMed Google Scholar * Angert, E. R. Alternatives to binary fission in bacteria. _Nat. Rev. Microbiol._ 3, 214–224 (2005). Article CAS PubMed Google Scholar * Tuson, H.
H. & Biteen, J. S. Unveiling the inner workings of live bacteria using super-resolution microscopy. _Anal. Chem._ 87, 42–63 (2015). Article CAS PubMed Google Scholar * Asano, S.,
Engel, B. D. & Baumeister, W. _In situ_ cryo-electron tomography: A post-reductionist approach to structural biology. _J. Mol. Biol._ 428, 332–343 (2015). Article PubMed CAS Google
Scholar * Busiek, K. K. & Margolin, W. Bacterial actin and tubulin homologs in cell growth and division. _Curr. Biol._ 25, 245–254 (2015). Article CAS Google Scholar * Grainge, I.
FtsK–a bacterial cell division checkpoint? _Mol. Microbiol._ 78, 1055–1057 (2010). Article CAS PubMed Google Scholar * Buss, J. et al. A multi-layered protein network stabilizes the
_Escherichia coli_ FtsZ-ring and modulates constriction dynamics. _PLoS Genet._ 11, e1005128 (2015). Article PubMed PubMed Central CAS Google Scholar Download references
ACKNOWLEDGEMENTS The authors gratefully acknowledge support from the US National Institute of General Medical Sciences (R01-GM61074 to W.M.) and the US National Institute of Research
Resources (S10RR029552) for the use of the 3D-SIM microscope. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Microbiology and Molecular Genetics, McGovern Medical School, 6431
Fannin Street, Houston, 77030, Texas, USA Daniel P. Haeusser & William Margolin * Biology Department, Canisius College, 2001 Main Street, Buffalo, 14208, New York, USA Daniel P. Haeusser
Authors * Daniel P. Haeusser View author publications You can also search for this author inPubMed Google Scholar * William Margolin View author publications You can also search for this
author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to William Margolin. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests.
POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 POWERPOINT SLIDE FOR FIG. 5 GLOSSARY * Cytokinesis The
splitting of the contents of a cell to make two cells. * Nucleoids The nucleus-like organized structures of bacterial chromosomes. * Mycelium A filamentous, branched network of multinucleate
cells growing on a surface. * Lipid II flippase A membrane protein that transfers lipid-linked peptidoglycan precursors from the inner leaflet of the cytoplasmic membrane to the outer
leaflet of the cytoplasmic membrane, so that they can be incorporated into the cell wall. * Sidewall The peptidoglycan layer in many rod-shaped bacteria that is active in elongating the cell
and comprises most of the straight wall of the cell with the exception of cell poles and the division septum. * Thermosensitive _ftsZ_ mutant A point mutation in the _ftsZ_ gene that
permits normal growth and division at 30 °C but stops division at 42 °C despite continued growth, resulting in filamentous, multinucleate cells (hence the term _fts_ for filamentous
temperature sensitive mutants). * Central metabolism Metabolic pathways, such as the tricarboxylic acid (TCA) cycle, that provide precursor metabolites for all other pathways that are
required for growth. * ClpXP A protein chaperone–protease machine that targets certain proteins for unfolding and degradation. * SOS response Induced by DNA damage, the SOS response is a
stress response that results in the expression of many genes that are important for the protection of chromosomal DNA. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE
THIS ARTICLE Haeusser, D., Margolin, W. Splitsville: structural and functional insights into the dynamic bacterial Z ring. _Nat Rev Microbiol_ 14, 305–319 (2016).
https://doi.org/10.1038/nrmicro.2016.26 Download citation * Published: 04 April 2016 * Issue Date: May 2016 * DOI: https://doi.org/10.1038/nrmicro.2016.26 SHARE THIS ARTICLE Anyone you share
the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative