- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
KEY POINTS * This review discusses how bacterial populations have been historically defined and classified, and how these definitions have been refined with the development of molecular
tools and whole-genome sequencing. * We discuss the impact of horizontal gene transfer and how the increased awareness of this process is influencing our understanding of bacterial
population structures. * We focus on the recent development of DNA microarray technology and its use as a method for high-throughput genome-wide strain comparisons. We give details of the
current achievements and limitations of this technique to assess the extent of genetic variability through a discussion of the current literature. We describe the application of this
technology to characterize isolates of _Helicobacter pylori_, _Streptococcus pneumoniae_, and _Salmonella enterica_ and _S. bongori_ species. * We propose how the information garnered from
comparative genomic microarray analyses can provide important insight into the molecular basis of the relationships that exist between bacteria and their hosts. ABSTRACT Sexual reproduction
and recombination are essential for the survival of most eukaryotic populations. Until recently, the impact of these processes on the structure of bacterial populations has been largely
overlooked. The advent of large-scale whole-genome sequencing and the concomitant development of molecular tools, such as microarray technology, facilitate the sensitive detection of
recombination events in bacteria. These techniques are revealing that bacterial populations are comprised of isolates that show a surprisingly wide spectrum of genetic diversity at the DNA
level. Our new awareness of this genetic diversity is increasing our understanding of population structures and of how these affect host–pathogen relationships. Access through your
institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print
issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to
local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT
BEING VIEWED BY OTHERS HORIZONTAL GENE TRANSFER AND ADAPTIVE EVOLUTION IN BACTERIA Article 12 November 2021 HOTSPOTS OF GENETIC CHANGE IN _YERSINIA PESTIS_ Article Open access 04 January
2025 ECOLOGY AND MOLECULAR TARGETS OF HYPERMUTATION IN THE GLOBAL MICROBIOME Article Open access 24 May 2021 REFERENCES * Van Belkum, A. et al. Role of genomic typing in taxonomy,
evolutionary genetics, and microbial epidemiology. _Clin. Microbiol. Rev._ 14, 547–560 (2001).A THOROUGH REVIEW THAT DETAILS CURRENT MICROBIAL NOMENCLATURE AND CONCEPTS OF EVOLUTIONARY AND
POPULATION-BASED GENETICS. IT REVIEWS VARIOUS MOLECULAR TECHNIQUES AND MAKES RECOMMENDATIONS FOR THEIR APPLICATION IN EPIDEMIOLOGY, TAXONOMY AND EVOLUTIONARY STUDIES. CAS PubMed PubMed
Central Google Scholar * Feil, E. J. & Spratt, B. G. Recombination and the population structures of baterial pathogens. _Annu. Rev. Microbiol._ 55, 561–590 (2001).THIS REVIEW ADDRESSES
THE IMPACT OF RECOMBINATION ON BACTERIAL POPULATIONS AND GIVES SPECIFIC FOCUS TO MULTILOCUS SEQUENCE TYPING. CAS PubMed Google Scholar * Milkman, R. Electrophoretic variation in
_Escherichia coli_ from natural sources. _Science_ 182, 1024–1026 (1973). CAS PubMed Google Scholar * Selander, R. K. Population genetics of pathogenic bacteria. _Microb. Pathog._ 3, 1–7
(1987). CAS PubMed Google Scholar * Beltran, P. Toward a population genetic analysis of _Salmonella_: genetic diversity and relationships among strains of serotypes _S. choleraesuis_, _S.
derby_, _S. dublin_, _S. enteritidis_, _S. heidelberg_, _S. infantis_, _S. newport_, and _S. typhimurium_. _Proc. Natl Acad. Sci. USA_ 85, 7753–7757 (1988). CAS PubMed PubMed Central
Google Scholar * Reeves, M. W. Clonal nature of _Salmonella_ typhi and its genetic relatedness to other Salmonellae as shown by multilocus enzyme electrophoresis, and proposal of
_Salmonella bongori_ comb. nov. _J. Clin. Microbiol._ 27, 313–320 (1989). CAS PubMed PubMed Central Google Scholar * Selander, R. K. Genetic population structure, clonal phylogeny, and
pathogenicity of _Salmonella_ paratyphi B. _Infect. Immun._ 58, 1891–1901 (1990). CAS PubMed PubMed Central Google Scholar * Selander, R. K. Evolutionary genetic relationships of clones
of _Salmonella_ serovars that cause human typhoid and other enteric fevers. _Infect. Immun._ 58, 2262–2275 (1990). CAS PubMed PubMed Central Google Scholar * Lefevre, J. C. DNA
fingerprinting of _Streptococcus pneumoniae_ strains by pulsed-field gel electrophoresis. _J. Clin. Microbiol._ 31, 2724–2728 (1993). CAS PubMed PubMed Central Google Scholar * Li, J. et
al. Recombinational basis of serovar diversity in _Salmonella enterica_. _Proc. Natl Acad. Sci. USA_ 91, 2552–2556 (1994). CAS PubMed PubMed Central Google Scholar * Spratt, B. G.
Resistance to antibiotics mediated by target alterations. _Science_ 264, 388–393 (1994). CAS PubMed Google Scholar * Musser, J. M. Molecular population genetic analysis of emerged
bacterial pathogens: selected insights. _Emerg. Infect. Dis._ 2, 1–17 (1996). CAS PubMed PubMed Central Google Scholar * Schloter, M. et al. Ecology and evolution of bacterial
microdiversity. _FEMS Microbiol. Rev._ 24, 647–660 (2000). CAS PubMed Google Scholar * Maynard Smith, J., Feil, E. J. & Smith, N. H. Population structure and evolutionary dynamics of
pathogenic bacteria. _Bioessays_ 22, 1115–1122 (2000).AN EXCELLENT REVIEW THAT DISCUSSES HOW MLEE, MLST AND ADVANCES IN NUCLEOTIDE SEQUENCING TECHNOLOGY GIVE A QUANTITATIVE ESTIMATE OF THE
IMPACT OF RECOMBINATION IN BACTERIA AND HOW THIS CONTRIBUTES TO OUR UNDERSTANDING OF BACTERIAL SPECIES DEFINITION. Google Scholar * Woese, C. R. Bacterial evolution. _Microbiol. Rev._ 51,
221–271 (1987). CAS PubMed PubMed Central Google Scholar * Ludwig, W. Bacterial phylogeny based on 16S and 23S rRNA sequence analysis. _FEMS Microbiol. Rev._ 15, 155–173 (1994). CAS
PubMed Google Scholar * Maiden, M. C. et al. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. _Proc. Natl
Acad. Sci. USA_ 95, 3140–3145 (1998).THIS PAPER INTRODUCES MULTILOCUS SEQUENCE TYPING AS A METHOD FOR TYPING MICROORGANISMS ON THE BASIS OF THE NUCLEOTIDE SEQUENCES OF A LIMITED NUMBER OF
GENETIC LOCI. CAS PubMed PubMed Central Google Scholar * Feil, E. J. Estimating the relative contributions of mutation and recombination to clonal diversification: a comparison between
_Neisseria meningitidis_ and _Streptococcus pneumoniae_. _Res. Microbiol._ 151, 465–469 (2000). CAS PubMed Google Scholar * Reid, S. D. et al. Parallel evolution of virulence in
pathogenic _Escherichia coli_. _Nature_ 406, 64–67 (2000). CAS PubMed Google Scholar * Wren, B. W. Microbial genome analysis: insights into virulence, host adaptation and evolution.
_Nature Rev. Genet._ 1, 30–39 (2000). CAS PubMed Google Scholar * Dobrindt, U. & Hacker, J. Whole genome plasticity in pathogenic bacteria. _Curr. Opin. Microbiol._ 4, 550–557 (2001).
CAS PubMed Google Scholar * Fitzgerald, J. R. & Musser, J. M. Evolutionary genomics of pathogenic bacteria. _Trends Microbiol._ 9, 547–553 (2001). CAS PubMed Google Scholar *
Graham, M. R. Toward a genome-scale understanding of group A _Streptococcus_ pathogenesis. _Curr. Opin. Microbiol._ 4, 65–70 (2001). CAS PubMed Google Scholar * Musser, J. M. Pneumococcal
research transformed. _N. Engl. J. Med._ 345, 1206–1207 (2001). CAS PubMed Google Scholar * Schaechter, M. _Escherichia coli_ and _Salmonella_ 2000: the view from here. _Microbiol. Mol.
Biol. Rev._ 65, 119–130 (2001). CAS PubMed PubMed Central Google Scholar * Edwards, R. A., Olsen, G. J. & Maloy, S. R. Comparative genomics of closely related Salmonellae. _Trends
Microbiol._ 10, 94–99 (2002). CAS PubMed Google Scholar * Schena, M., Shalon, D., Davis, R. W. & Brown, P. O. Quantitative monitoring of gene expression patterns with a complementary
DNA microarray. _Science_ 270, 467–470 (1995). CAS PubMed Google Scholar * Cummings, C. A. & Relman, D. A. Using DNA microarrays to study host–microbe interactions. _Emerg. Infect.
Dis._ 6, 513–525 (2000). CAS PubMed PubMed Central Google Scholar * Diehn, M. & Relman, D. A. Comparing functional genomic datasets: lessons from DNA microarray analyses of
host–pathogen interactions. _Curr. Opin. Microbiol._ 4, 95–101 (2001). CAS PubMed Google Scholar * Lucchini, S., Thompson, A. & Hinton, J. C. Microarrays for microbiologists.
_Microbiology_ 147, 1403–1414 (2001). CAS PubMed Google Scholar * Sassetti, C. M., Boyd, D. H. & Rubin, E. J. Comprehensive identification of conditionally essential genes in
mycobacteria. _Proc. Natl Acad. Sci. USA_ 98, 12712–12717 (2001). CAS PubMed PubMed Central Google Scholar * Schoolnik, G. K. The accelerating convergence of genomics and microbiology.
_Genome Biol. Online_ 2, REPORTS4009 (2001). * Behr, M. A. et al. Comparative genomics of BCG vaccines by whole-genome DNA microarray. _Science_ 284, 1520–1523 (1999). CAS PubMed Google
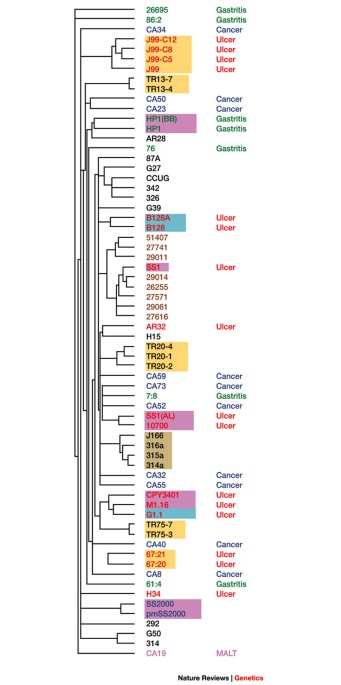
Scholar * Salama, N. et al. A whole-genome microarray reveals genetic diversity among _Helicobacter pylori_ strains. _Proc. Natl Acad. Sci. USA_ 97, 14668–14673 (2000).THIS STUDY PROVIDED
THE FIRST EVIDENCE OF THE EXTENT OF GENETIC DIVERSITY IN BACTERIA USING MICROARRAY ANALYSIS. CAS PubMed PubMed Central Google Scholar * Wu, L. et al. Development and evaluation of
functional gene arrays for detection of selected genes in the environment. _Appl. Environ. Microbiol._ 67, 5780–5790 (2001).THIS CRUCIAL STUDY DESCRIBES CONDITIONS FOR EXAMINING GENE
COMPOSITION IN NATURAL MICROBIAL COMMUNITIES USING MICROARRAY ANALYSIS. CAS PubMed PubMed Central Google Scholar * Cho, J. C. & Tiedje, J. M. Bacterial species determination from
DNA–DNA hybridization by using genome fragments and DNA microarrays. _Appl. Environ. Microbiol._ 67, 3677–3682 (2001).A METHOD BASED ON RANDOM GENOME FRAGMENTS AND DNA MICROARRAY TECHNOLOGY
THAT REVEALS TAXONOMIC RELATIONSHIPS BETWEEN BACTERIAL STRAINS. CAS PubMed PubMed Central Google Scholar * Dorrell, N. et al. Whole genome comparison of _Campylobacter jejuni_ human
isolates using a low-cost microarray reveals extensive genetic diversity. _Genome Res._ 11, 1706–1715 (2001). CAS PubMed PubMed Central Google Scholar * Bjorkholm, B. et al. Comparison
of genetic divergence and fitness between two subclones of _Helicobacter pylori_. _Infect. Immun._ 69, 7832–7838 (2001).BY USING MICROARRAY ANALYSIS, THE AUTHORS DETECTED GENETIC CHANGES
BETWEEN CLINICAL ISOLATES COLLECTED FROM A SINGLE PATIENT, ALLUDING TO THE POTENTIAL FOR _IN VIVO_ SUB-SPECIES DEVELOPMENT. CAS PubMed PubMed Central Google Scholar * Hurt, R. A. et al.
Simultaneous recovery of RNA and DNA from soils and sediments. _Appl. Environ. Microbiol._ 67, 4495–4503 (2001). CAS PubMed PubMed Central Google Scholar * Janssen, P. J., Audit, B.
& Ouzounis, C. A. Strain-specific genes of _Helicobacter pylori_: distribution, function and dynamics. _Nucleic Acids Res._ 29, 4395–4404 (2001). CAS PubMed PubMed Central Google
Scholar * Israel, D. A. et al. _Helicobacter pylori_ genetic diversity within the gastric niche of a single human host. _Proc. Natl Acad. Sci. USA_ 98, 14625–14630 (2001).THIS STUDY USED
MICROARRAYS TO EXAMINE THE EXTENT AND TYPES OF GENETIC CHANGE IN A BACTERIAL PATHOGEN THAT OCCUR DURING LONG-TERM HOST COLONIZATION AND HOW THESE CORRELATE WITH CLINICAL OUTCOMES OF
INFECTION. CAS PubMed PubMed Central Google Scholar * Israel, D. A. et al. _Helicobacter pylori_ strain-specific differences in genetic content, identified by microarray, influence host
inflammatory responses. _J. Clin. Invest._ 107, 611–620 (2001).MICROARRAY TECHNOLOGY WAS USED TO INVESTIGATE _H. PYLORI_ GENETIC DIVERSITY AND TO CORRELATE THIS WITH DISEASE DEVELOPMENT AND
SEVERITY. CAS PubMed PubMed Central Google Scholar * Hakenbeck, R. et al. Mosaic genes and mosaic chromosomes: intra- and interspecies genomic variation of _Streptococcus pneumoniae_.
_Infect. Immun._ 69, 2477–2486 (2001).THESE AUTHORS USED AN AFFYMETRIX HIGH-DENSITY OLIGONUCLEOTIDE ARRAY TO EXAMINE THE GENETIC RELATEDNESS OF 20 CLINICAL _S. PNEUMONIAE_ ISOLATES, 5
_STREPTOCOCCUS MITIS_ ISOLATES AND 4 _STREPTOCOCCUS ORALIS_ ISOLATES. CAS PubMed PubMed Central Google Scholar * Tettelin, H. et al. Complete genome sequence of a virulent isolate of
_Streptococcus pneumoniae_. _Science_ 293, 498–506 (2001). CAS PubMed Google Scholar * Fitzgerald, J. R. et al. Evolutionary genomics of _Staphylococcus aureus_: insights into the origin
of methicillin-resistant strains and the toxic shock syndrome epidemic. _Proc. Natl Acad. Sci. USA_ 98, 8821–8826 (2001). CAS PubMed PubMed Central Google Scholar * Murray, A. E. et al.
DNA/DNA hybridization to microarrays reveals gene-specific differences between closely related microbial genomes. _Proc. Natl Acad. Sci. USA_ 98, 9853–9858 (2001). CAS PubMed PubMed
Central Google Scholar * Dziejman, M. et al. Comparative genomic analysis of _Vibrio cholerae_: genes that correlate with cholera endemic and pandemic disease. _Proc. Natl Acad. Sci. USA_
99, 1556–1561 (2002). CAS PubMed PubMed Central Google Scholar * Smoot, J. C. et al. Genome sequence and comparative microarray analysis of serotype M18 groupA _Streptococcus_ strains
associated with acute rheumatic fever outbreaks. _Proc. Natl Acad. Sci. USA_ 99, 4668–4673 (2002). CAS PubMed PubMed Central Google Scholar * Wotherspoon, A. C. et al. _Helicobacter
pylori_-associated gastritis and primary B-cell gastric lymphoma. _Lancet_ 338, 1175–1176 (1991). CAS PubMed Google Scholar * Montecucco, C. Living dangerously: how _Helicobacter pylori_
survives in the human stomach. _Nature Rev. Mol. Cell Biol._ 2, 457–466 (2001). CAS Google Scholar * Tummuru, M. K. et al. Cloning and expression of a high-molecular-mass major antigen of
_Helicobacter pylori_: evidence of linkage to cytotoxin production. _Infect. Immun._ 61, 1799–1809 (1993). CAS PubMed PubMed Central Google Scholar * Covacci, A. et al. Molecular
characterization of the 128-kDa immunodominant antigen of _Helicobacter pylori_ associated with cytotoxicity and duodenal ulcer. _Proc. Natl Acad. Sci. USA_ 90, 5791–5795 (1993). CAS PubMed
PubMed Central Google Scholar * Censini, S. et al. _cag_, a pathogenicity island of _Helicobacter pylori_, encodes type I-specific and disease-associated virulence factors. _Proc. Natl
Acad. Sci. USA_ 93, 14648–14653 (1996). CAS PubMed PubMed Central Google Scholar * Backert, S. et al. Translocation of the _Helicobacter pylori_ CagA protein in gastric epithelial cells
by a type IV secretion apparatus. _Cell. Microbiol._ 2, 155–164 (2000). CAS PubMed Google Scholar * Odenbreit, S. et al. Translocation of _Helicobacter pylori_ CagA into gastric
epithelial cells by type IV secretion. _Science_ 287, 1497–1500 (2000). CAS PubMed Google Scholar * Segal, E. D. et al. Altered states: involvement of phosphorylated CagA in the induction
of host cellular growth changes by _Helicobacter pylori_. _Proc. Natl Acad. Sci. USA_ 96, 14559–14564 (1999). CAS PubMed PubMed Central Google Scholar * Stein, M., Rappuoli, R. &
Covacci, A. Tyrosine phosphorylation of the _Helicobacter pylori_ CagA antigen after _cag_-driven host cell translocation. _Proc. Natl Acad. Sci. USA_ 97, 1263–1268 (2000). CAS PubMed
PubMed Central Google Scholar * Akopyanz, N. et al. PCR-based RFLP analysis of DNA sequence diversity in the gastric pathogen _Helicobacter pylori_. _Nucleic Acids Res._ 20, 6221–6225
(1992). CAS PubMed PubMed Central Google Scholar * Akopyanz, N. et al. DNA diversity among clinical isolates of _Helicobacter pylori_ detected by PCR-based RAPD fingerprinting. _Nucleic
Acids Res._ 20, 5137–5142 (1992). CAS PubMed PubMed Central Google Scholar * Alm, R. A. et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen
_Helicobacter pylori_. _Nature_ 397, 176–180 (1999). PubMed Google Scholar * Alm, R. A. & Trust, T. J. Analysis of the genetic diversity of _Helicobacter pylori_: the tale of two
genomes. _J. Mol. Med._ 77, 834–846 (1999). CAS PubMed Google Scholar * Tomb, J. F. et al. The complete genome sequence of the gastric pathogen _Helicobacter pylori_. _Nature_ 388,
539–547 (1997). CAS PubMed Google Scholar * Pellegrini, M. et al. Assigning protein functions by comparative genome analysis: protein phylogenetic profiles. _Proc. Natl Acad. Sci. USA_
96, 4285–4288 (1999). CAS PubMed PubMed Central Google Scholar * Gerhard, M. et al. Clinical relevance of the _Helicobacter pylori_ gene for blood-group antigen-binding adhesin. _Proc.
Natl Acad. Sci. USA_ 96, 12778–12783 (1999). CAS PubMed PubMed Central Google Scholar * Ilver, D. et al. _Helicobacter pylori_ adhesin binding fucosylated histo-blood group antigens
revealed by retagging. _Science_ 279, 373–377 (1998). CAS PubMed Google Scholar * Parsonnet, J. et al. _Helicobacter pylori_ infection and the risk of gastric carcinoma. _N. Engl. J.
Med._ 325, 1127–1131 (1991). CAS PubMed Google Scholar * Sharma, S. A. et al. Interleukin-8 response of gastric epithelial cell lines to _Helicobacter pylori_ stimulation _in vitro_.
_Infect. Immun._ 63, 1681–1687 (1995). CAS PubMed PubMed Central Google Scholar * Tummuru, M. K., Sharma, S. A. & Blaser, M. J. _Helicobacter pylori picB_, a homologue of the
_Bordetella pertussis_ toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. _Mol. Microbiol._ 18, 867–876 (1995). CAS PubMed Google Scholar * Hoskins,
J. et al. Genome of the bacterium _Streptococcus pneumoniae_ strain R6. _J. Bacteriol._ 183, 5709–5717 (2001). CAS PubMed PubMed Central Google Scholar * Hall, L. M. et al. Genetic
relatedness within and between serotypes of _Streptococcus pneumoniae_ from the United Kingdom: analysis of multilocus enzyme electrophoresis, pulsed-field gel electrophoresis, and
antimicrobial resistance patterns. _J. Clin. Microbiol._ 34, 853–859 (1996). CAS PubMed PubMed Central Google Scholar * Spratt, B. G. & Maiden, M. C. Bacterial population genetics,
evolution and epidemiology. _Phil. Trans. R. Soc. Lond. B_ 354, 701–710 (1999). CAS Google Scholar * Gray, B. M. et al. Serotypes of _Streptococcus pneumoniae_ causing disease. _J. Infect.
Dis._ 140, 979–983 (1979). CAS PubMed Google Scholar * Gray, B. M. et al. Clinical and epidemiologic studies of pneumococcal infection in children. _Pediatr. Infect. Dis._ 5, 201–207
(1986). CAS PubMed Google Scholar * Orange, M. & Gray, B. M. Pneumococcal serotypes causing disease in children in Alabama. _Pediatr. Infect. Dis. J._ 12, 244–246 (1993). CAS PubMed
Google Scholar * Baumler, A. J. et al. Evolution of host adaptation in _Salmonella enterica_. _Infect. Immun._ 66, 4579–4587 (1998). CAS PubMed PubMed Central Google Scholar * Conner,
C. P. et al. Differential patterns of acquired virulence genes distinguish _Salmonella_ strains. _Proc. Natl Acad. Sci. USA_ 95, 4641–4645 (1998). CAS PubMed PubMed Central Google
Scholar * Folkesson, A. et al. Multiple insertions of fimbrial operons correlate with the evolution of _Salmonella_ serovars responsible for human disease. _Mol. Microbiol._ 33, 612–622
(1999). CAS PubMed Google Scholar * Townsend, S. M. et al. _Salmonella enterica_ serovar Typhi possesses a unique repertoire of fimbrial gene sequences. _Infect. Immun._ 69, 2894–2901
(2001). CAS PubMed PubMed Central Google Scholar * Baumler, A. J., Hargis, B. M. & Tsolis, R. M. Tracing the origins of _Salmonella_ outbreaks. _Science_ 287, 50–52 (2000). CAS
PubMed Google Scholar * Kingsley, R. A. & Baumler, A. J. Host adaptation and the emergence of infectious disease: the _Salmonella_ paradigm. _Mol. Microbiol._ 36, 1006–1014 (2000). CAS
PubMed Google Scholar * Rabsch, W. et al. Competitive exclusion of _Salmonella enteritidis_ by _Salmonella gallinarum_ in poultry. _Emerg. Infect. Dis._ 6, 443–448 (2000). CAS PubMed
PubMed Central Google Scholar * Woolhouse, M. E., Taylor, L. H. & Haydon, D. T. Population biology of multihost pathogens. _Science_ 292, 1109–1112 (2001). CAS PubMed Google Scholar
* McClelland, M. et al. Complete genome sequence of _Salmonella enterica_ serovar Typhimurium LT2. _Nature_ 413, 852–856 (2001). CAS PubMed Google Scholar * Parkhill, J. et al. Complete
genome sequence of a multiple drug resistant _Salmonella enterica_ serovar Typhi CT18. _Nature_ 413, 848–852 (2001). CAS PubMed Google Scholar * Boyd, E. F. et al. Molecular genetic
relationships of the Salmonellae. _Appl. Environ. Microbiol._ 62, 804–808 (1996). CAS PubMed PubMed Central Google Scholar * Smith, B. P. et al. Aromatic-dependent _Salmonella_
typhimurium as modified live vaccines for calves. _Am. J. Vet. Res._ 45, 59–66 (1984). CAS PubMed Google Scholar * Boyd, E. F. et al. _Salmonella_ reference collection B (SARB): strains
of 37 serovars of subspecies I. _J. Gen. Microbiol._ 139, 1125–1132 (1993). CAS PubMed Google Scholar * Figueroa-Bossi, N. et al. Variable assortment of prophages provides a transferable
repertoire of pathogenic determinants in _Salmonella_. _Mol. Microbiol._ 39, 260–271 (2001). CAS PubMed Google Scholar * Woodward, M. J., McLaren, I. & Wray, C. Distribution of
virulence plasmids within Salmonellae. _J. Gen. Microbiol._ 135, 503–511 (1989). CAS PubMed Google Scholar * Tinge, S. A. & Curtiss, R. Conservation of _Salmonella_ typhimurium
virulence plasmid maintenance regions among _Salmonella_ serovars as a basis for plasmid curing. _Infect. Immun._ 58, 3084–3092 (1990). CAS PubMed PubMed Central Google Scholar * Chiu,
C. H. et al. Prevalence of the virulence plasmids of nontyphoid _Salmonella_ in the serovars isolated from humans and their association with bacteremia. _Microbiol. Immunol._ 43, 899–903
(1999). CAS PubMed Google Scholar * Ochman, H. & Groisman, E. A. Distribution of pathogenicity islands in _Salmonella_ spp. _Infect. Immun._ 64, 5410–5412 (1996). CAS PubMed PubMed
Central Google Scholar * Xu, Q. et al. Identification of type II restriction and modification systems in _Helicobacter pylori_ reveals their substantial diversity among strains. _Proc.
Natl Acad. Sci. USA_ 97, 9671–9676 (2000). CAS PubMed PubMed Central Google Scholar * Aras, R. A. et al. Regulation of the _Hpy_II restriction-modification system of _Helicobacter
pylori_ by gene deletion and horizontal reconstitution. _Mol. Microbiol._ 42, 369–382 (2001). CAS PubMed Google Scholar * Davies, J. Origins and evolution of antibiotic resistance.
_Microbiologia_ 12, 9–16 (1996). CAS PubMed Google Scholar * Brown, J. R. & Doolittle, W. F. Archaea and the prokaryote-to-eukaryote transition. _Microbiol. Mol. Biol. Rev._ 61,
456–502 (1997). CAS PubMed PubMed Central Google Scholar * Doolittle, R. F. & Handy, J. Evolutionary anomalies among the aminoacyl-tRNA synthetases. _Curr. Opin. Genet. Dev._ 8,
630–636 (1998). CAS PubMed Google Scholar * Doolittle, W. F. & Logsdon, J. M. Jr. Archaeal genomics: do Archaea have a mixed heritage? _Curr. Biol._ 8, R209–R211 (1998). CAS PubMed
Google Scholar * Doolittle, W. F. Phylogenetic classification and the universal tree. _Science_ 284, 2124–2129 (1999). CAS PubMed Google Scholar * Koonin, E. V. et al. Comparison of
archaeal and bacterial genomes: computer analysis of protein sequences predicts novel functions and suggests a chimeric origin for the Archaea. _Mol. Microbiol._ 25, 619–637 (1997). CAS
PubMed Google Scholar * Lawrence, J. G. & Ochman, H. Molecular archaeology of the _Escherichia coli_ genome. _Proc. Natl Acad. Sci. USA_ 95, 9413–9417 (1998). CAS PubMed PubMed
Central Google Scholar * Nelson, K. E. et al. Evidence for lateral gene transfer between Archaea and bacteria from genome sequence of _Thermotoga maritima_. _Nature_ 399, 323–329 (1999).
CAS PubMed Google Scholar * Ochman, H., Lawrence, J. G. & Groisman, E. A. Lateral gene transfer and the nature of bacterial innovation. _Nature_ 405, 299–304 (2000). CAS PubMed
Google Scholar * Doolittle, W. F. Lateral genomics. _Trends Cell Biol._ 9, M5–M8 (1999). CAS PubMed Google Scholar * McNulty, C. A. The discovery of _Campylobacter_-like organisms.
_Curr. Top. Microbiol. Immunol._ 241, 1–9 (1999). CAS PubMed Google Scholar * Marshall, B. J. & Warren, J. R. Unidentified curved bacilli in the stomach of patients with gastritis and
peptic ulceration. _Lancet_ 1, 1311–1315 (1984). CAS PubMed Google Scholar * Suerbaum, S. Genetic variability within _Helicobacter pylori_. _Ijmm Int. J. Med. Microbiol._ 290, 175–181
(2000). CAS PubMed Google Scholar * Kelly, D. Infectious ulcers: not hurry, worry and curry? _Microbiol. Today_ 28, 188–189 (2001). Google Scholar * Musher, D. M. Infections caused by
_Streptococcus pneumoniae_: clinical spectrum, pathogenesis, immunity, and treatment. _Clin. Infect. Dis._ 14, 801–807 (1992). CAS PubMed Google Scholar * Hausdorff, W. P. et al. Which
pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. _Clin. Infect. Dis._ 30, 100–121 (2000). CAS PubMed Google Scholar
* McCarty, M. in _Microbiology, Including Immunology and Molecular Genetics_ (eds Davis, B. D., Dulbecco, R., Eisen, H. N. & Ginsberg, H. S.) 607–622 (Harper & Row, Philadelphia,
1980). Google Scholar * Neufeld, F. Uber die agglutina der pneumokokken und uber die theorien der agglutination. _Z. Hyg. Infekt-Kr_ 40, 54–72 (1902). Google Scholar * Kim, J. O. et al.
Relationship between cell surface carbohydrates and intrastrain variation on opsonophagocytosis of _Streptococcus pneumoniae_. _Infect. Immun._ 67, 2327–2333 (1999). CAS PubMed PubMed
Central Google Scholar * White, P. B. _Great Britain Medical Research Council Special Report: No. 103_ (Her Majesty's Stationery Office, London, 1926). Google Scholar * Crosa, J. H.
et al. Molecular relationships among the Salmonellae. _J. Bacteriol._ 115, 307–315 (1973). CAS PubMed PubMed Central Google Scholar * Scherer, C. A. & Miller, S. I. in _Principles of
Bacterial Pathogenesis_ (ed. Groisman, E. A.) 266–316 (Academic, San Diego, 2001). Google Scholar * Anriany, Y. A. et al. _Salmonella enterica_ serovar Typhimurium DT104 displays a rugose
phenotype. _Appl. Environ. Microbiol._ 67, 4048–4056 (2001). CAS PubMed PubMed Central Google Scholar * McGee, L. et al. Nomenclature of major antimicrobial-resistant clones of
_Streptococcus pneumoniae_ defined by the Pneumococcal Molecular Epidemiology Network. _J. Clin. Microbiol._ 39, 2565–2571 (2001). CAS PubMed PubMed Central Google Scholar Download
references ACKNOWLEDGEMENTS We thank our colleagues G. Dougan and S. Baker from the Imperial College of Science, Technology and Medicine, London, for _Salmonella_ strains and sharing data;
A. Covacci, Chiron-Biocine, Italy; J. Gordon, Washington University, Missouri; J. Parsonette, Stanford University, California; R. Peek Jr, Vanderbilt University, Tennessee; J. Solnick,
University of California at Davis, for providing _H. pylori_ clinical isolates; L. McGee from the Pneumococcal Diseases Research Unit at the South African Institute for Medical Research,
Johannesburg, for the _S. pneumoniae_ strains; and A. Kawale and S. Censini for technical assistance, C. Kim for developing analytical tools for microarray analysis and thoughtful
discussion, and S. Reid, J. R. Fitzgerald and members of the Falkow Laboratory for critical reviews of the manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of
Microbiology and Immunology, Stanford University School of Medicine, 299 Campus Drive, Fairchild D 037, Stanford, 94305-5402, California, USA Elizabeth A. Joyce, Kaman Chan & Stanley
Falkow * Division of Human Biology, Frederick Hutchinson Cancer Research Center, 1100 Fairview Avenue North, Seattle, 98109, Washington, USA Nina R. Salama Authors * Elizabeth A. Joyce View
author publications You can also search for this author inPubMed Google Scholar * Kaman Chan View author publications You can also search for this author inPubMed Google Scholar * Nina R.
Salama View author publications You can also search for this author inPubMed Google Scholar * Stanley Falkow View author publications You can also search for this author inPubMed Google
Scholar CORRESPONDING AUTHOR Correspondence to Elizabeth A. Joyce. RELATED LINKS RELATED LINKS FURTHER INFORMATION CLUSTER program Genome Entry Database at NCBI Multilocus sequence typing
NCBI GenBank Database _Salmonella_.org _Salmonella_ Reference Collections B and C Supplementary Table 6 from REF. 44 The Institute for Genomic Research (TIGR) GLOSSARY * CONJUGATION The
transfer of DNA from a donor cell to a recipient cell that is mediated by direct cell–cell contact. * TRANSFORMATION The uptake of DNA by a bacterium from the surrounding environment. *
TRANSDUCTION Virus- or phage-mediated introduction into a cell of a DNA fragment that is derived from a different cell. * ARCHAEA A kingdom of unicellular microorganisms, many members of
which can survive extreme environmental conditions, such as temperatures >100 °C, extremely alkaline or acid environs, and highly osmotic conditions. * RIBOTYPING A technique used to
determine genetic and evolutionary relationships between organisms. Oligonucleotide probes targeted to highly conserved domains of coding sequences of ribosomal RNA are amplified and the
products are visualized by gel electrophoresis banding patterns are compared with known species and strains to determine organism relatedness. * ATROPHIC GASTRITIS Chronic inflammation of
the stomach, accompanied by atrophy of the mucous membrane and destruction of the peptic glands. * DUODENUM The first portion of the small intestine, extending from the pylorus (the
posterior end of the stomach) to the jejunum (the next portion of the small intestine). * ADENOCARCINOMA A form of malignant cancer that arises from the glandular epithelium. *
SEROVAR/SEROTYPE A group of intimately related microorganisms distinguished by a common set of antigenic determinants that are expressed on the cell surface. * CHEMOKINES Small molecules
that have a central role in inflammatory responses and trigger migration and activation of phagocytic cells and lymphocytes. * CLUSTER ANALYSIS A mathematical algorithm that organizes a set
of items according to their similarity. For example, genes can be clustered according to their similarity in pattern of expression. * INSERTION SEQUENCES Small, mobile nucleotide sequences
found in the genomes of many bacterial populations. * ADHESIVE FIMBRIAE Hair-like structures that project from the surface of some bacteria. They are involved in adhesion of bacterial cells
to surfaces, and can be important in bacterial virulence. * OTITIS MEDIA Infection and inflammation of the middle ear space and ear drum. * GRAM REACTION A differential stain that separates
bacteria into two groups, Gram positive and Gram negative, on the basis of the biochemical composition of their cell wall. * DIPLOCOCCUS Any of a variety of encapsulated bacteria (as the
pneumococcus) that usually occur in pairs. * CAPSULE A thick gel-like material generally composed of hydrophilic polysaccharide that surrounds the cell wall of Gram-positive or Gram-negative
bacteria. It can contribute to pathogenicity by inhibiting phagocytosis of the bacteria by the macrophages of the host. * ENTEROBACTERIACEAE A large family of Gram-negative bacilli that
inhabit the large intestine of mammals. * AGGLUTINATED The aggregation of particulate antigen by antibodies. * TISSUE TROPISM Tissue-specific bacterial adherence and colonization due to a
restricted distribution of receptor structures on certain host-cell surfaces and not on others. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Joyce,
E., Chan, K., Salama, N. _et al._ Redefining bacterial populations: a post-genomic reformation. _Nat Rev Genet_ 3, 462–473 (2002). https://doi.org/10.1038/nrg820 Download citation * Issue
Date: 01 June 2002 * DOI: https://doi.org/10.1038/nrg820 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative






