- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
KEY POINTS * The key bottlenecks for small RNA drugs that harness RNA interference (RNAi) for selective gene knockdown _in vivo_ include their delivery across the plasma membrane and their
release from endosomes into the cytosol. * Small interfering RNA (siRNA)-based drugs can now be delivered into the cytosol of hepatocytes to suppress gene expression in the liver. * Recent
siRNA clinical trials show durable and potent gene silencing in the liver, with manageable toxicity for a handful of disease targets. * The most effective strategies for gene knockdown in
the liver use second-generation lipid nanoparticles or GalNAc-conjugated siRNAs that are taken up by the asialoglycoprotein receptor, which is exclusively expressed by hepatocytes. *
Achieving gene knockdown outside the liver is still clinically unproven. The most attractive strategies use topical administration of siRNAs to accessible tissue sites, such as the skin, eye
or mucosa, or use siRNAs that are covalently linked to an RNA aptamer that binds with high affinity to a cell surface receptor selectively expressed on cells being targeted for gene
knockdown. * Methods that are being developed to deliver siRNAs therapeutically may eventually prove to be useful for delivering other nucleic acid therapeutics, including antisense
oligonucleotides, mRNAs for gene expression, or CRISPR–Cas9 (clustered regularly interspaced short palindromic repeats–CRISPR-associated 9) for gene editing. ABSTRACT Small interfering RNAs
(siRNAs), which downregulate gene expression guided by sequence complementarity, can be used therapeutically to block the synthesis of disease-causing proteins. The main obstacle to siRNA
drugs — their delivery into the target cell cytosol — has been overcome to allow suppression of liver gene expression. Here, we review the results of recent clinical trials of siRNA
therapeutics, which show efficient and durable gene knockdown in the liver, with signs of promising clinical outcomes and little toxicity. We also discuss the barriers to more widespread
applications that target tissues besides the liver and the most promising avenues to overcome them. Access through your institution Buy or subscribe This is a preview of subscription
content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue
Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL
ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS RNAI-BASED DRUG DESIGN: CONSIDERATIONS
AND FUTURE DIRECTIONS Article 03 April 2024 RNA INTERFERENCE IN THE ERA OF NUCLEIC ACID THERAPEUTICS Article 26 February 2024 THERAPEUTIC SIRNA: STATE OF THE ART Article Open access 19 June
2020 REFERENCES * Zamecnik, P. C. & Stephenson, M. L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. _Proc. Natl Acad. Sci. USA_
75, 280–284 (1978). CAS PubMed Google Scholar * Stephenson, M. L. & Zamecnik, P. C. Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide. _Proc.
Natl Acad. Sci. USA_ 75, 285–288 (1978). FIRST DEMONSTRATION OF THE POSSIBILITY OF INHIBITING GENE EXPRESSION USING OLIGONUCLEOTIDES. CAS PubMed Google Scholar * Kole, R., Krainer, A. R.
& Altman, S. RNA therapeutics: beyond RNA interference and antisense oligonucleotides. _Nat. Rev. Drug Discov._ 11, 125–140 (2012). CAS PubMed PubMed Central Google Scholar *
Bennett, C. F. & Swayze, E. E. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. _Annu. Rev. Pharmacol. Toxicol._ 50, 259–293
(2010). CAS PubMed Google Scholar * Sharma, V. K., Sharma, R. K. & Singh, S. K. Antisense oligonucleotides: modifications and clinical trials. _Med. Chem. Commun._ 5, 1454–1471
(2014). CAS Google Scholar * Raal, F. J. et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial
hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. _Lancet_ 375, 998–1006 (2010). CAS PubMed Google Scholar * Fire, A. et al. Potent and specific genetic
interference by double-stranded RNA in _Caenorhabditis elegans_. _Nature_ 391, 806–811 (1998). INITIAL REPORT DESCRIBING MEDIATION OF RNAI BY DOUBLE-STRANDED RNAS. CAS PubMed Google
Scholar * Elbashir, S. M. et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. _Nature_ 411, 494–498 (2001). DEMONSTRATION THAT EXOGENOUSLY SUPPLIED
SHORT DOUBLE-STRANDED RNAS CAN SPECIFICALLY AND EFFICIENTLY INHIBIT GENE EXPRESSION IN MAMMALIAN CELLS. CAS PubMed Google Scholar * Song, E. et al. RNA interference targeting Fas protects
mice from fulminant hepatitis. _Nat. Med._ 9, 347–351 (2003). FIRST PROOF OF PRINCIPLE THAT SIRNAS CAN BE HARNESSED TO TREAT DISEASE IN AN _IN VIVO_ MODEL. CAS PubMed Google Scholar *
Morrissey, D. V. et al. Activity of stabilized short interfering RNA in a mouse model of hepatitis B virus replication. _Hepatology_ 41, 1349–1356 (2005). CAS PubMed Google Scholar *
Chiu, Y.-L. & Rana, T. M. siRNA function in RNAi: a chemical modification analysis. _RNA_ 9, 1034–1048 (2003). CAS PubMed PubMed Central Google Scholar * Morrissey, D. V. et al.
Potent and persistent _in vivo_ anti-HBV activity of chemically modified siRNAs. _Nat. Biotechnol._ 23, 1002–1007 (2005). FIRST DEMONSTRATION THAT SIRNAS CAN BE CHEMICALLY MODIFIED TO
ATTENUATE NUCLEASE DEGRADATION AND IMMUNE STIMULATION TO ALLOW FOR EFFICIENT LIPID-BASED SYSTEMIC ADMINISTRATION. CAS PubMed Google Scholar * Judge, A. D., Bola, G., Lee, A. C. H. &
MacLachlan, I. Design of noninflammatory synthetic siRNA mediating potent gene silencing _in vivo_. _Mol. Ther._ 13, 494–505 (2006). CAS PubMed Google Scholar * Jackson, A. L. et al.
Position-specific chemical modification of siRNAs reduces 'off-target' transcript silencing. _RNA_ 12, 1197–1205 (2006). CAS PubMed PubMed Central Google Scholar * Bartlett, D.
W. & Davis, M. E. Insights into the kinetics of siRNA-mediated gene silencing from live-cell and live-animal bioluminescent imaging. _Nucleic Acids Res._ 34, 322–333 (2006). CAS PubMed
PubMed Central Google Scholar * Gilleron, J. et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. _Nat. Biotechnol._
31, 638–646 (2013). CAS PubMed Google Scholar * Wittrup, A. et al. Visualizing lipid-formulated siRNA release from endosomes and target gene knockdown. _Nat. Biotechnol._
http://dx.doi.org/10.1038/nbt.3298 (2015). * Kanasty, R., Dorkin, J. R., Vegas, A. & Anderson, D. Delivery materials for siRNA therapeutics. _Nat. Mater._ 12, 967–977 (2013). CAS PubMed
Google Scholar * Zimmermann, T. S. et al. RNAi-mediated gene silencing in non-human primates. _Nature_ 441, 111–114 (2006). FIRST REPORT OF POTENT RNAI-MEDIATED GENE SILENCING IN
NON-HUMAN PRIMATES AFTER SYSTEMIC ADMINISTRATION OF SIRNA FORMULATED IN LNPS. CAS PubMed Google Scholar * Akinc, A. et al. A combinatorial library of lipid-like materials for delivery of
RNAi therapeutics. _Nat. Biotechnol._ 26, 561–569 (2008). CAS PubMed PubMed Central Google Scholar * Semple, S. C. et al. Rational design of cationic lipids for siRNA delivery. _Nat.
Biotechnol._ 28, 172–176 (2010). CAS PubMed Google Scholar * Shi, B. et al. Biodistribution of small interfering RNA at the organ and cellular levels after lipid nanoparticle-mediated
delivery. _J. Histochem. Cytochem._ 59, 727–740 (2011). CAS PubMed PubMed Central Google Scholar * Akinc, A. et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous
ligand-based mechanisms. _Mol. Ther._ 18, 1357–1364 (2010). CAS PubMed PubMed Central Google Scholar * Judge, A. D. et al. Confirming the RNAi-mediated mechanism of action of siRNA-based
cancer therapeutics in mice. _J. Clin. Invest._ 119, 661–673 (2009). CAS PubMed PubMed Central Google Scholar * Bartlett, D. W., Su, H., Hildebrandt, I. J., Weber, W. A. & Davis, M.
E. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality _in vivo_ imaging. _Proc. Natl Acad. Sci. USA_ 104, 15549–15554
(2007). CAS PubMed Google Scholar * Song, E. et al. Antibody mediated _in vivo_ delivery of small interfering RNAs via cell-surface receptors. _Nat. Biotechnol._ 23, 709–717 (2005). CAS
PubMed Google Scholar * Peer, D., Zhu, P., Carman, C. V., Lieberman, J. & Shimaoka, M. Selective gene silencing in activated leukocytes by targeting siRNAs to the integrin lymphocyte
function-associated antigen-1. _Proc. Natl Acad. Sci. USA_ 104, 4095–4100 (2007). CAS PubMed Google Scholar * Peer, D., Park, E. J., Morishita, Y., Carman, C. V. & Shimaoka, M.
Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. _Science_ 319, 627–630 (2008). CAS PubMed PubMed Central Google Scholar * McNamara, J. O.
et al. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. _Nat. Biotechnol._ 24, 1005–1015 (2006). CAS PubMed Google Scholar * Berezhnoy, A., Castro, I., Levay, A., Malek,
T. R. & Gilboa, E. Aptamer-targeted inhibition of mTOR in T cells enhances antitumor immunity. _J. Clin. Invest._ 124, 188–197 (2014). CAS PubMed Google Scholar * Wheeler, L. A. et
al. Inhibition of HIV transmission in human cervicovaginal explants and humanized mice using CD4 aptamer-siRNA chimeras. _J. Clin. Invest._ 121, 2401–2412 (2011). CAS PubMed PubMed Central
Google Scholar * Davis, M. E. et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. _Nature_ 464, 1067–1070 (2010). CAS PubMed PubMed
Central Google Scholar * Nair, J. K. et al. Multivalent _N_-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. _J. Am. Chem.
Soc._ 136, 16958–16961 (2014). DESCRIPTION OF TRIANTENNARY GALNAC-CONJUGATED SIRNA FOR LIVER-TARGETED GENE KNOCKDOWN. CAS PubMed Google Scholar * Wong, S. C. et al. Co-injection of a
targeted, reversibly masked endosomolytic polymer dramatically improves the efficacy of cholesterol-conjugated small interfering RNAs _in vivo_. _Nucleic Acid Ther._ 22, 380–390 (2012). CAS
PubMed PubMed Central Google Scholar * Kortylewski, M. et al. _In vivo_ delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. _Nat.
Biotechnol._ 27, 925–932 (2009). CAS PubMed PubMed Central Google Scholar * Soutschek, J. et al. Therapeutic silencing of an endogenous gene by systemic administration of modified
siRNAs. _Nature_ 432, 173–178 (2004). CAS PubMed Google Scholar * Biessen, E. A. et al. Synthesis of cluster galactosides with high affinity for the hepatic asialoglycoprotein receptor.
_J. Med. Chem._ 38, 1538–1546 (1995). CAS PubMed Google Scholar * Matsuda, S. et al. siRNA conjugates carrying sequentially assembled trivalent _N_-acetylgalactosamine linked through
nucleosides elicit robust gene silencing _in vivo_ in hepatocytes. _ACS Chem. Biol._ 10, 1181–1187 (2015). CAS PubMed Google Scholar * Rajeev, K. G. et al. Hepatocyte-specific delivery of
siRNAs conjugated to novel non-nucleosidic trivalent _N_-acetylgalactosamine elicits robust gene silencing _in vivo_. _ChemBioChem_ 16, 903–908 (2015). CAS PubMed Google Scholar *
Manoharan, M. GalNAc-siRNA with enhanced stabilization chemistry: ESC-GalNAc-siRNA. _Alnylam_ [online], (2014). Google Scholar * Thurston, T. L. M., Wandel, M. P., von Muhlinen, N.,
Foeglein, Á. & Randow, F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. _Nature_ 482, 414–418 (2012). CAS PubMed PubMed Central Google
Scholar * Boussif, O., Zanta, M. A. & Behr, J. P. Optimized galenics improve _in vitro_ gene transfer with cationic molecules up to 1000-fold. _Gene Ther._ 3, 1074–1080 (1996). CAS
PubMed Google Scholar * Rozema, D. B. et al. Dynamic PolyConjugates for targeted _in vivo_ delivery of siRNA to hepatocytes. _Proc. Natl Acad. Sci. USA_ 104, 12982–12987 (2007). CAS
PubMed Google Scholar * Cohen, J. C., Boerwinkle, E., Mosley, T. H. Jr & Hobbs, H. H. Sequence variations in _PCSK9_, low LDL, and protection against coronary heart disease. _N. Engl.
J. Med._ 354, 1264–1272 (2006). CAS PubMed Google Scholar * Sorensen, B. et al. A subcutaneously administered RNAi therapeutic (ALN-AT3) targeting antithrombin for treatment of
hemophilia: interim Phase 1 study results in healthy volunteers and patients with hemophilia A or B. Oral Poster Abstract 693. _56_th _American Society of Hematology_ [online], (2014).
Google Scholar * Tabernero, J. et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. _Cancer Discov._ 3, 406–417
(2013). CAS PubMed Google Scholar * Reynolds, A. et al. Rational siRNA design for RNA interference. _Nat. Biotechnol._ 22, 326–330 (2004). CAS PubMed Google Scholar * Elbashir, S. M.,
Harborth, J., Weber, K. & Tuschl, T. Analysis of gene function in somatic mammalian cells using small interfering RNAs. _Methods_ 26, 199–213 (2002). CAS PubMed Google Scholar *
Jackson, A. L. et al. Expression profiling reveals off-target gene regulation by RNAi. _Nat. Biotechnol._ 21, 635–637 (2003). CAS PubMed Google Scholar * Jackson, A. L. et al. Widespread
siRNA 'off-target' transcript silencing mediated by seed region sequence complementarity. _RNA_ 12, 1179–1187 (2006). CAS PubMed PubMed Central Google Scholar * Birmingham, A.
et al. 3′ UTR seed matches, but not overall identity, are associated with RNAi off-targets. _Nat. Methods_ 3, 199–204 (2006). CAS PubMed Google Scholar * Huang, L. et al. Efficient and
specific gene knockdown by small interfering RNAs produced in bacteria. _Nat. Biotechnol._ 31, 350–356 (2013). CAS PubMed PubMed Central Google Scholar * Judge, A. D. et al.
Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. _Nat. Biotechnol._ 23, 457–462 (2005). CAS PubMed Google Scholar * Kleinman, M. E. et al.
Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. _Nature_ 452, 591–597 (2008). CAS PubMed PubMed Central Google Scholar * Hornung, V. et al. Sequence-specific
potent induction of IFN-α by short interfering RNA in plasmacytoid dendritic cells through TLR7. _Nat. Med._ 11, 263–270 (2005). CAS PubMed Google Scholar * Poeck, H. et al.
5′-triphosphate-siRNA: turning gene silencing and Rig-I activation against melanoma. _Nat. Med._ 14, 1256–1263 (2008). CAS PubMed Google Scholar * Roberts, T. L., Sweet, M. J., Hume, D.
A. & Stacey, K. J. Cutting edge: species-specific TLR9-mediated recognition of CpG and non-CpG phosphorothioate-modified oligonucleotides. _J. Immunol._ 174, 605–608 (2005). CAS PubMed
Google Scholar * Kedmi, R., Ben-Arie, N. & Peer, D. The systemic toxicity of positively charged lipid nanoparticles and the role of Toll-like receptor 4 in immune activation.
_Biomaterials_ 31, 6867–6875 (2010). CAS PubMed Google Scholar * Landesman-Milo, D. & Peer, D. Toxicity profiling of several common RNAi-based nanomedicines: a comparative study.
_Drug Deliv. Transl. Res._ 4, 96–103 (2014). CAS PubMed Google Scholar * Maier, M. A. et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of
RNAi therapeutics. _Mol. Ther._ 21, 1570–1578 (2013). CAS PubMed PubMed Central Google Scholar * Rusconi, C. Phase 3 evaluation of revolixys kit: study summary and lessons learned.
_Celebrating the 25th Anniversary of Selex, ASGCT meeting May 10–12, 2015_, New Orleans (2015). Google Scholar * Coelho, T. et al. Safety and efficacy of RNAi therapy for transthyretin
amyloidosis. _N. Engl. J. Med._ 369, 819–829 (2013). FIRST DEMONSTRATION OF HIGHLY POTENT SIRNA-MEDIATED GENE KNOCKDOWN IN HUMANS. CAS PubMed Google Scholar * Degenhardt, Y. &
Lampkin, T. Targeting Polo-like kinase in cancer therapy. _Clin. Cancer Res._ 16, 384–389 (2010). CAS PubMed Google Scholar * Geisbert, T. W. et al. Postexposure protection of non-human
primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study. _Lancet_ 375, 1896–1905 (2010). CAS PubMed Google Scholar * Fitzgerald, K. et al. Effect
of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised,
single-blind, placebo-controlled, phase 1 trial. _Lancet_ 383, 60–68 (2014). CAS PubMed Google Scholar * Adams, D. et al. Phase 2 open-label extension study of patisiran, an RNAi
therapeutic for the treatment of familial amyloidotic polyneuropathy. _Alnylam_ [online], (2014) Google Scholar * Zimmermann, T. et al. Phase I first-in-human trial of ALN-TTRsc, a novel
RNA interference therapeutic for the treatment of familial amyloidotic cardiomyopathy (FAC). _Alnylam_ [online], (2013). Google Scholar * Akinc, A. A. Subcutaneously administered
investigational RNAi therapeutic (ALN-AT3) targeting antithrombin for treatment of hemophilia: interim Phase 1 study results in healthy volunteers and hemophilia A and B subjects. _Alnylam_
[online], (2015). Google Scholar * [No authors listed.] RNAi Roundtable: ALN-PCSsc for the treatment of hypercholesterolemia. _Alnylam_ [online], (2014). * Wooddell, C. I. et al.
Hepatocyte-targeted RNAi therapeutics for the treatment of chronic hepatitis B virus infection. _Mol. Ther._ 21, 973–985 (2013). CAS PubMed PubMed Central Google Scholar * Yuen, M.-F. et
al. Phase II, dose ranging study of ARC-520, a siRNA-based therapeutic, in patients with chronic hepatitis B virus infection. _American Association for the Study of Liver Diseases_
[online], (2014). Google Scholar * [No authors listed.] Arrowhead begins Phase 1 trial of ARC-AAT for treatment of liver disease associated with alpha-1 antitrypsin deficiency. _Arrowhead
Research_ [online], (2015). * Byrne, M. et al. Novel hydrophobically modified asymmetric RNAi compounds (sd-rxRNA) demonstrate robust efficacy in the eye. _J. Ocul. Pharmacol. Ther._ 29,
855–864 (2013). CAS PubMed Google Scholar * [No authors listed.] Clinical trials — overview. _RXI Pharmaceuticals_ [online] * Tolcher, A. W. et al. Safety and activity of DCR-MYC, a
first-in-class Dicer-substrate small interfering RNA (DsiRNA) targeting MYC, in a phase I study in patients with advanced solid tumors. _J. Clin. Oncol._ 33, 915_suppl 11006 (2015). Google
Scholar * Dudek, H. et al. Knockdown of β-catenin with dicer-substrate siRNAs reduces liver tumor burden _in vivo_. _Mol. Ther._ 22, 92–101 (2014). CAS PubMed Google Scholar *
Schultheis, B. et al. First-in-human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. _J. Clin. Oncol._ 32, 4141–4148 (2014). CAS
PubMed Google Scholar * Wu, Y. et al. Durable protection from herpes simplex virus-2 transmission following intravaginal application of siRNAs targeting both a viral and host gene. _Cell
Host Microbe_ 5, 84–94 (2009). CAS PubMed PubMed Central Google Scholar * Nakayama, T. et al. Harnessing a physiologic mechanism for siRNA delivery with mimetic lipoprotein particles.
_Mol. Ther._ 20, 1582–1589 (2012). CAS PubMed PubMed Central Google Scholar * Alvarez-Erviti, L. et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes.
_Nat. Biotechnol._ 29, 341–345 (2011). CAS PubMed Google Scholar * Kumar, P. et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. _Cell_ 134, 577–586
(2008). CAS PubMed PubMed Central Google Scholar * Yao, Y.-D. et al. Targeted delivery of PLK1-siRNA by ScFv suppresses _Her2_+ breast cancer growth and metastasis. _Sci. Transl Med._ 4,
130ra48 (2012). PubMed Google Scholar * Cuellar, T. L. et al. Systematic evaluation of antibody-mediated siRNA delivery using an industrial platform of THIOMAB-siRNA conjugates. _Nucleic
Acids Res._ 43, 1189–1203 (2015). CAS PubMed Google Scholar * Rozema, D. B. et al. Protease-triggered siRNA delivery vehicles. _J. Control. Release_ 209, 57–66 (2015). CAS PubMed Google
Scholar * Meade, B. R. et al. Efficient delivery of RNAi prodrugs containing reversible charge-neutralizing phosphotriester backbone modifications. _Nat. Biotechnol._ 32, 1256–1261 (2014).
CAS PubMed PubMed Central Google Scholar * Warren, L. et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA.
_Cell Stem Cell_ 7, 618–630 (2010). CAS PubMed PubMed Central Google Scholar * Yin, H. et al. Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. _Nat.
Biotechnol._ 32, 551–553 (2014). CAS PubMed PubMed Central Google Scholar * Goemans, N. M. et al. Systemic administration of PRO051 in Duchenne's muscular dystrophy. _N. Engl. J.
Med._ 364, 1513–1522 (2011). CAS PubMed Google Scholar * Janssen, H. L. A. et al. Treatment of HCV infection by targeting microRNA. _N. Engl. J. Med._ 368, 1685–1694 (2013). CAS PubMed
Google Scholar * Bhat, B. et al. RG-101, a GalNAC-conjugated anti-miR employing aunique mechanism of action by targeting host factor microRNA-122 (miR-122), demonstrates potent activity and
reduction of HCV in preclinical studies. _Hepatology_ 58, 1393A (2013). Google Scholar * Hata, A. & Lieberman, J. Dysregulation of microRNA biogenesis and gene silencing in cancer.
_Sci. Signal._ 8, re3 (2015). PubMed Google Scholar * Daige, C. L. et al. Systemic delivery of a miR34a mimic as a potential therapeutic for liver cancer. _Mol. Cancer Ther._ 13, 2352–2360
(2014). CAS PubMed Google Scholar * Prakash, T. P. et al. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary _N_-acetyl galactosamine improves potency
10-fold in mice. _Nucleic Acids Res._ 42, 8796–8807 (2014). CAS PubMed PubMed Central Google Scholar * Rozema, D. B., Ekena, K., Lewis, D. L., Loomis, A. G. & Wolff, J. A.
Endosomolysis by masking of a membrane-active agent (EMMA) for cytoplasmic release of macromolecules. _Bioconjug. Chem._ 14, 51–57 (2003). CAS PubMed Google Scholar * Gilboa-Geffen, A. et
al. Gene knockdown by EpCAM aptamer–siRNA chimeras suppresses epithelial breast cancers and their tumor-initiating cells. _Mol. Cancer Ther._ (in the press). Download references
ACKNOWLEDGEMENTS This work was supported by the Swedish Research Council (A.W.) and the US National Institutes of Health (NIH) grant CA139444 (to J.L.). AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Boston Children's Hospital and Department of Pediatrics, Program in Cellular and Molecular Medicine, Harvard Medical School, Boston, 02115, Massachusetts, USA Anders
Wittrup & Judy Lieberman * Department of Clinical Sciences, Section for Oncology and Pathology, Lund University, 221 85, Lund, Sweden Anders Wittrup Authors * Anders Wittrup View author
publications You can also search for this author inPubMed Google Scholar * Judy Lieberman View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING
AUTHOR Correspondence to Judy Lieberman. ETHICS DECLARATIONS COMPETING INTERESTS J.L. is on the Scientific Advisory Board of Alnylam Pharmaceuticals. A.W. declares no competing interests.
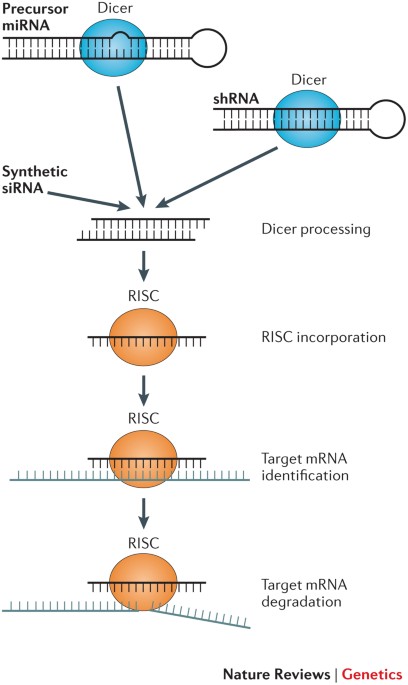
POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR TABLE 1 GLOSSARY * RNA interference (RNAi). An endogenous gene silencing mechanism, present in virtually all eukaryotic
cells, by which short double-stranded RNA molecules induce translational inhibition and/or degradation of mRNAs containing partially complementary sequences. * Gene knockdown An experimental
technique used to reduce gene expression using sequence-specific oligonucleotides, typically by RNA interference (RNAi) or antisense mechanisms. * RNA-induced silencing complex (RISC). The
catalytic effector complex of RNA interference (RNAi)-mediated gene silencing. The RISC is a multiprotein complex that incorporates one strand of a small interfering RNA (siRNA) or microRNA.
* Aptamers Oligonucleotides (DNA or RNA) selected to bind with high affinity to defined structures. * MicroRNAs (miRNAs). Endogenous, ~21-nucleotide-long, imperfectly paired double-stranded
RNA molecules present in both plants and animals that guide the silencing of a multitude of genes bearing partially complementary sequences. * Endosomal escape The process of cytosolic
entry of small interfering RNAs (siRNAs) from a vesicular compartment, following initial endocytosis of the siRNA (and delivery vehicle) into the target cell. RIGHTS AND PERMISSIONS Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wittrup, A., Lieberman, J. Knocking down disease: a progress report on siRNA therapeutics. _Nat Rev Genet_ 16, 543–552 (2015).
https://doi.org/10.1038/nrg3978 Download citation * Published: 18 August 2015 * Issue Date: September 2015 * DOI: https://doi.org/10.1038/nrg3978 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative


:max_bytes(150000):strip_icc():focal(999x0:1001x2)/randi-2000-b4bc9dc46b6c403d94ed914bd682abde.jpg)


:max_bytes(150000):strip_icc():focal(645x317:647x319)/dime-doe-102124-1-116fea1dba7d4a67be7ccb6e8f08ee76.jpg)



