- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
KEY POINTS * Global approaches are defining the full complement of proteins with RNA-binding capacity in living cells. * RNA packaging by proteins begins during transcription and determines
the fate of the RNA. * RNA-binding proteins (RBPs) can act as 'molecular rulers', sorting RNAs according to their length. * RBPs can link sequential and non-sequential processing
steps. * A future challenge is to define the composition of individual mRNPs at different stages of remodelling. ABSTRACT mRNA is packaged into ribonucleoprotein particles called mRNPs. A
multitude of RNA-binding proteins as well as a host of associated proteins participate in the fate of mRNA from transcription and processing in the nucleus to translation and decay in the
cytoplasm. Methodological innovations in cell biology and genome-wide high-throughput approaches have revealed an unexpected diversity of mRNA-associated proteins and unforeseen
interconnections between mRNA-processing steps. Recent insights into mRNP formation _in vivo_ have also highlighted the importance of mRNP packaging, which can sort RNAs on the basis of
their length and determine mRNA fate through alternative mRNP assembly, processing and export pathways. Access through your institution Buy or subscribe This is a preview of subscription
content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue
Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL
ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS NUCLEAR MRNA DECAY: REGULATORY NETWORKS
THAT CONTROL GENE EXPRESSION Article 18 April 2024 MRNA RECOGNITION AND PACKAGING BY THE HUMAN TRANSCRIPTION–EXPORT COMPLEX Article 05 April 2023 CYTOPLASMIC MRNA DECAY AND QUALITY CONTROL
MACHINERIES IN EUKARYOTES Article 27 January 2025 REFERENCES * Fischer, U., Englbrecht, C. & Chari, A. Biogenesis of spliceosomal small nuclear ribonucleoproteins. _Wiley Interdiscip_.
_Rev. RNA_ 2, 718–731 (2011). CAS PubMed Google Scholar * Watkins, N. J. & Bohnsack, M. T. The box C/D and H/ACA snoRNPs: key players in the modification, processing and the dynamic
folding of ribosomal RNA. _Wiley Interdiscip. Rev. RNA_ 3, 397–414 (2012). CAS PubMed Google Scholar * Keene, J. D. RNA regulons: coordination of post-transcriptional events. _Nature Rev.
Genet._ 8, 533–543 (2007). CAS PubMed Google Scholar * Ascano, M., Hafner, M., Cekan, P., Gerstberger, S. & Tuschl, T. Identification of RNA–protein interaction networks using
PAR-CLIP. _Wiley Interdiscip_. _Rev. RNA_ 3, 159–177 (2012). CAS PubMed Google Scholar * Anko, M. L. & Neugebauer, K. M. RNA–protein interactions _in vivo_: global gets specific.
_Trends Biochem. Sci._ 37, 255–262 (2012). PubMed Google Scholar * Moore, M. J. & Proudfoot, N. J. Pre-mRNA processing reaches back to transcription and ahead to translation. _Cell_
136, 688–700 (2009). CAS PubMed Google Scholar * Schoenberg, D. R. & Maquat, L. E. Regulation of cytoplasmic mRNA decay. _Nature Rev. Genet._ 13, 246–259 (2012). CAS PubMed Google
Scholar * König, J., Zarnack, K., Luscombe, N. M. & Ule, J. Protein–RNA interactions: new genomic technologies and perspectives. _Nature Rev. Genet._ 13, 77–83 (2011). Google Scholar *
Tutucci, E. & Stutz, F. Keeping mRNPs in check during assembly and nuclear export. _Nature Rev. Mol. Cell Biol._ 12, 377–384 (2011). CAS Google Scholar * Grunwald, D. & Singer, R.
H. Multiscale dynamics in nucleocytoplasmic transport. _Curr. Opin. Cell Biol._ 24, 100–106 (2012). PubMed Google Scholar * Castello, A. et al. Insights into RNA biology from an atlas of
mammalian mRNA-binding proteins. _Cell_ 149, 1393–1406 (2012). CAS PubMed Google Scholar * Baltz, A. G. et al. The mRNA-bound proteome and its global occupancy profile on protein-coding
transcripts. _Mol. Cell_ 46, 674–690 (2012). REFERENCES 11 AND 12 INTRODUCE NEW METHODS TO IDENTIFY RNA-BINDING PROTEINS GLOBALLY IN HUMAN CELL LINES, TO PROVIDE A COMPREHENSIVE ATLAS OF
PROTEINS THAT CAN BIND TO POLYADENYLATED RNAS AND TO IDENTIFY NOVEL RNA-BINDING DOMAINS. CAS PubMed Google Scholar * Mitchell, S. F., Jain, S., She, M. & Parker, R. Global analysis of
yeast mRNPs. _Nature Struct. Mol. Biol._ 20, 127–133 (2013). CAS Google Scholar * Kishore, S., Luber, S. & Zavolan, M. Deciphering the role of RNA-binding proteins in the
post-transcriptional control of gene expression. _Brief_. _Funct. Genom._ 9, 391–404 (2010). CAS Google Scholar * Cui, X. A., Zhang, H. & Palazzo, A. F. p180 promotes the
ribosome-independent localization of a subset of mRNA to the endoplasmic reticulum. _PLoS Biol._ 10, e1001336 (2012). CAS PubMed PubMed Central Google Scholar * Mackereth, C. D. &
Sattler, M. Dynamics in multi-domain protein recognition of RNA. _Curr. Opin. Struct. Biol._ 22, 287–296 (2012). CAS PubMed Google Scholar * Wahl, M. C., Will, C. L. & Luhrmann, R.
The spliceosome: design principles of a dynamic RNP machine. _Cell_ 136, 701–718 (2009). CAS PubMed Google Scholar * MacRae, I. J., Zhou, K. & Doudna, J. A. Structural determinants of
RNA recognition and cleavage by Dicer. _Nature Struct. Mol. Biol._ 14, 934–940 (2007). CAS Google Scholar * Lamichhane, R., Solem, A., Black, W. & Rueda, D. Single-molecule FRET of
protein–nucleic acid and protein–protein complexes: surface passivation and immobilization. _Methods_ 52, 192–200 (2010). CAS PubMed PubMed Central Google Scholar * Konig, J. et al.
iCLIP reveals the function of hnRNP particles in splicing at individual nucleotide resolution. _Nature Struct. Mol. Biol._ 17, 909–915 (2010). INTRODUCING A NEW METHOD TO IDENTIFY DIRECT
BINDING SITES OF RNA-BINDING PROTEINS WITH A HIGH RESOLUTION, TERMED ICLIP, THE AUTHORS OF THIS PAPER INDICATE THAT HNRNPC IS CRUCIAL FOR THE CO-TRANSCRIPTIONAL PACKAGING OF MOST RNAS AND
PROVIDE A FUNCTIONAL LINK TO ALTERNATIVE SPLICING. Google Scholar * Kumar, A. & Pederson, T. Comparison of proteins bound to heterogeneous nuclear RNA and messenger RNA in HeLa cells.
_J. Mol. Biol._ 96, 353–365 (1975). CAS PubMed Google Scholar * Beyer, A. L., Christensen, M. E., Walker, B. W. & LeStourgeon, W. M. Identification and characterization of the
packaging proteins of core 40S hnRNP particles. _Cell_ 11, 127–138 (1977). CAS PubMed Google Scholar * Derman, E., Goldberg, S. & Darnell, J. E. Jr. hnRNA in HeLa cells: distribution
of transcript sizes estimated from nascent molecule profile. _Cell_ 9, 465–472 (1976). CAS PubMed Google Scholar * Mor, A. et al. Dynamics of single mRNP nucleocytoplasmic transport and
export through the nuclear pore in living cells. _Nature Cell Biol._ 12, 543–552 (2010). CAS PubMed Google Scholar * Gorlach, M., Burd, C. G., Portman, D. S. & Dreyfuss, G. The hnRNP
proteins. _Mol. Biol. Rep._ 18, 73–78 (1993). CAS PubMed Google Scholar * Dreyfuss, G., Choi, Y. D. & Adam, S. A. The ribonucleoprotein structures along the pathway of mRNA formation.
_Endocr. Res._ 15, 441–474 (1989). CAS PubMed Google Scholar * Weighardt, F., Biamonti, G. & Riva, S. The roles of heterogeneous nuclear ribonucleoproteins (hnRNP) in RNA metabolism.
_BioEssays_ 18, 747–756 (1996). CAS PubMed Google Scholar * McAfee, J. G., Soltaninassab, S. R., Lindsay, M. E. & LeStourgeon, W. M. Proteins C1 and C2 of heterogeneous nuclear
ribonucleoprotein complexes bind RNA in a highly cooperative fashion: support for their contiguous deposition on pre-mRNA during transcription. _Biochemistry_ 35, 1212–1222 (1996). CAS
PubMed Google Scholar * McAfee, J. G., Shahied-Milam, L., Soltaninassab, S. R. & LeStourgeon, W. M. A major determinant of hnRNP C protein binding to RNA is a novel bZIP-like RNA
binding domain. _RNA_ 2, 1139–1152 (1996). CAS PubMed PubMed Central Google Scholar * Huang, M. et al. The C-protein tetramer binds 230 to 240 nucleotides of pre-mRNA and nucleates the
assembly of 40S heterogeneous nuclear ribonucleoprotein particles. _Mol. Cell. Biol._ 14, 518–533 (1994). CAS PubMed PubMed Central Google Scholar * Neugebauer, K. M. Please hold—the
next available exon will be right with you. _Nature Struct. Mol. Biol._ 13, 385–386 (2006). CAS Google Scholar * Bentley, D. L. Rules of engagement: co-transcriptional recruitment of
pre-mRNA processing factors. _Curr. Opin. Cell Biol._ 17, 251–256 (2005). CAS PubMed Google Scholar * Listerman, I., Sapra, A. K. & Neugebauer, K. M. Cotranscriptional coupling of
splicing factor recruitment and precursor messenger RNA splicing in mammalian cells. _Nature Struct. Mol. Biol._ 13, 815–822 (2006). CAS Google Scholar * Wetterberg, I., Zhao, J., Masich,
S., Wieslander, L. & Skoglund, U. _In situ_ transcription and splicing in the Balbiani ring 3 gene. _EMBO J._ 20, 2564–2574 (2001). CAS PubMed PubMed Central Google Scholar *
Aguilera, A. & Garcia-Muse, T. R loops: from transcription byproducts to threats to genome stability. _Mol. Cell_ 46, 115–124 (2012). CAS PubMed Google Scholar * Dye, M. J., Gromak,
N. & Proudfoot, N. J. Exon tethering in transcription by RNA polymerase II. _Mol. Cell_ 21, 849–859 (2006). CAS PubMed Google Scholar * Martinez-Contreras, R. et al. hnRNP proteins
and splicing control. _Adv. Exp. Med. Biol._ 623, 123–147 (2007). PubMed Google Scholar * Vargas, D. Y. et al. Single-molecule imaging of transcriptionally coupled and uncoupled splicing.
_Cell_ 147, 1054–1065 (2011). CAS PubMed PubMed Central Google Scholar * McCloskey, A. Taniguchi, I., Shinmyozu, K. & Ohno, M. hnRNP C tetramer measures RNA length to classify RNA
polymerase II transcripts for export. _Science_ 335, 1643–1646 (2012). THIS PAPER DESCRIBES A NEW MECHANISM IN WHICH POL II TRANSCRIPTS ARE SORTED ACCORDING TO THEIR LENGTH PRIOR TO NUCLEAR
EXPORT AND IDENTIFIED HNRNPC AS THE KEY PLAYER. CAS PubMed Google Scholar * Merz, C., Urlaub, H., Will, C. L. & Luhrmann, R. Protein composition of human mRNPs spliced _in vitro_ and
differential requirements for mRNP protein recruitment. _RNA_ 13, 116–128 (2007). CAS PubMed PubMed Central Google Scholar * Anko, M. L., Morales, L., Henry, I., Beyer, A. &
Neugebauer, K. M. Global analysis reveals SRp20- and SRp75-specific mRNPs in cycling and neural cells. _Nature Struct. Mol. Biol._ 17, 962–970 (2010). Google Scholar * Singh, G. et al. The
cellular EJC interactome reveals higher-order mRNP structure and an EJC-SR protein nexus. _Cell_ 151, 750–764 (2012). THIS PAPER PRESENTS COMPELLING EVIDENCE SUGGESTING THAT THE EJC AND SR
PROTEINS COOPERATE IN THE PACKAGING AND COMPACTION OF MATURE MRNPS FOR EFFICIENT NUCLEAR EXPORT. CAS PubMed PubMed Central Google Scholar * Sauliere, J. et al. CLIP-seq of eIF4AIII
reveals transcriptome-wide mapping of the human exon junction complex. _Nature Struct. Mol. Biol._ 19, 1124–1131 (2012). CAS Google Scholar * Bjork, P. et al. Specific combinations of SR
proteins associate with single pre-messenger RNAs _in vivo_ and contribute different functions. _J. Cell Biol._ 184, 555–568 (2009). PubMed PubMed Central Google Scholar * Walsh, M. J.,
Hautbergue, G. M. & Wilson, S. A. Structure and function of mRNA export adaptors. _Biochem. Soc. Trans._ 38, 232–236 (2010). CAS PubMed Google Scholar * Sapra, A. K. et al. SR protein
family members display diverse activities in the formation of nascent and mature mRNPs _in vivo_. _Mol. Cell_ 34, 179–190 (2009). CAS PubMed Google Scholar * Lin, S., Xiao, R., Sun, P.,
Xu, X. & Fu, X. D. Dephosphorylation-dependent sorting of SR splicing factors during mRNP maturation. _Mol. Cell_ 20, 413–425 (2005). CAS PubMed Google Scholar * Caceres, J. F.,
Screaton, G. R. & Krainer, A. R. A specific subset of SR proteins shuttles continuously between the nucleus and the cytoplasm. _Genes Dev._ 12, 55–66 (1998). CAS PubMed PubMed Central
Google Scholar * Delestienne, N. et al. The splicing factor ASF/SF2 is associated with TIA-1-related/TIA-1-containing ribonucleoproteic complexes and contributes to post-transcriptional
repression of gene expression. _FEBS J._ 277, 2496–2514 (2010). CAS PubMed Google Scholar * Anko, M. L. et al. The RNA-binding landscapes of two SR proteins reveal unique functions and
binding to diverse RNA classes. _Genome Biol._ 13, R17 (2012). PubMed PubMed Central Google Scholar * Huang, Y. & Steitz, J. A. SRprises along a messenger's journey. _Mol. Cell_
17, 613–615 (2005). CAS PubMed Google Scholar * Erkmann, J. A., Sanchez, R., Treichel, N., Marzluff, W. F. & Kutay, U. Nuclear export of metazoan replication-dependent histone mRNAs
is dependent on RNA length and is mediated by TAP. _RNA_ 11, 45–58 (2005). CAS PubMed PubMed Central Google Scholar * Strasser, K. et al. TREX is a conserved complex coupling
transcription with messenger RNA export. _Nature_ 417, 304–308 (2002). PubMed Google Scholar * Reed, R. & Cheng, H. TREX, SR proteins and export of mRNA. _Curr. Opin. Cell Biol._ 17,
269–273 (2005). CAS PubMed Google Scholar * Dias, A. P., Dufu, K., Lei, H. & Reed, R. A role for TREX components in the release of spliced mRNA from nuclear speckle domains. _Nature
Commun._ 1, 97 (2010). Google Scholar * Katahira, J., Inoue, H., Hurt, E. & Yoneda, Y. Adaptor Aly and co-adaptor Thoc5 function in the Tap-p15-mediated nuclear export of HSP70 mRNA.
_EMBO J._ 28, 556–567 (2009). CAS PubMed PubMed Central Google Scholar * Lei, H., Dias, A. P. & Reed, R. Export and stability of naturally intronless mRNAs require specific coding
region sequences and the TREX mRNA export complex. _Proc. Natl Acad. Sci. USA_ 108, 17985–17990 (2011). CAS PubMed PubMed Central Google Scholar * Palazzo, A. F. et al. The signal
sequence coding region promotes nuclear export of mRNA. _PLoS Biol._ 5, e322 (2007). PubMed PubMed Central Google Scholar * Palazzo, A. F. & Akef, A. Nuclear export as a key arbiter
of 'mRNA identity' in eukaryotes. _Biochim. Biophys. Acta_ 1819, 566–577 (2012). CAS PubMed Google Scholar * Cheng, H. et al. Human mRNA export machinery recruited to the 5′ end
of mRNA. _Cell_ 127, 1389–1400 (2006). CAS PubMed Google Scholar * Nojima, T., Hirose, T., Kimura, H. & Hagiwara, M. The interaction between cap-binding complex and RNA export factor
is required for intronless mRNA export. _J. Biol. Chem._ 282, 15645–15651 (2007). CAS PubMed Google Scholar * Sullivan, K. D., Mullen, T. E., Marzluff, W. F. & Wagner, E. J.
Knockdown of SLBP results in nuclear retention of histone mRNA. _RNA_ 15, 459–472 (2009). CAS PubMed PubMed Central Google Scholar * Narita, T. et al. NELF interacts with CBC and
participates in 3′ end processing of replication-dependent histone mRNAs. _Mol. Cell_ 26, 349–365 (2007). CAS PubMed Google Scholar * Wickramasinghe, V. O. et al. mRNA export from
mammalian cell nuclei is dependent on GANP. _Curr. Biol._ 20, 25–31 (2010). CAS PubMed PubMed Central Google Scholar * Jani, D. et al. Functional and structural characterization of the
mammalian TREX-2 complex that links transcription with nuclear messenger RNA export. _Nucleic Acids Res._ 40, 4562–4573 (2012). CAS PubMed PubMed Central Google Scholar * Proudfoot, N.
J. Ending the message: poly(A) signals then and now. _Genes Dev._ 25, 1770–1782 (2011). CAS PubMed PubMed Central Google Scholar * Lou, H., Neugebauer, K. M., Gagel, R. F. & Berget,
S. M. Regulation of alternative polyadenylation by U1 snRNPs and SRp20. _Mol. Cell. Biol._ 18, 4977–4985 (1998). CAS PubMed PubMed Central Google Scholar * Berg, M. G. et al. U1 snRNP
determines mRNA length and regulates isoform expression. _Cell_ 150, 53–64 (2012). THIS PAPER ESTABLISHES U1 SNRNP AS A MOLECULAR RULER AND DESCRIBES HOW U1 SNRNP LEVELS INFLUENCE THE LENGTH
OF TRANSCRIPTS THROUGH SUPPRESSION OF PCPA. THE AUTHORS DEMONSTRATE THE PHYSIOLOGICAL IMPORTANCE OF THIS ROLE OF U1 SNRNP IN ACTIVATED NEURONS. CAS PubMed PubMed Central Google Scholar
* Kaida, D. et al. U1 snRNP protects pre-mRNAs from premature cleavage and polyadenylation. _Nature_ 468, 664–668 (2010). IN THIS STUDY, THE AUTHORS DISCOVERED A NEW SPLICING-INDEPENDENT
FUNCTION OF U1 SNRNP AS A SUPPRESSOR OF ALTERNATIVE POLYADENYLATION. THE AUTHORS SHOW THAT U1 SNRNP PROTECTS PRE-MRNAS FROM PCPA BY BINDING TO INAPPROPRIATE POLY(A) SITES PRESENT WITHIN
INTRONS OF PRE-MRNAS. CAS PubMed PubMed Central Google Scholar * Eckmann, C. R., Rammelt, C. & Wahle, E. Control of poly(A) tail length. _Wiley Interdiscip_. _Rev. RNA_ 2, 348–361
(2011). CAS PubMed Google Scholar * Keller, R. W. et al. The nuclear poly(A) binding protein, PABP2, forms an oligomeric particle covering the length of the poly(A) tail. _J. Mol. Biol._
297, 569–583 (2000). CAS PubMed Google Scholar * Lemay, J.-F., Lemieux, C., St-André, O. & Bachand, F. Crossing the borders: poly(A)-binding proteins working on both sides of the
fence. _RNA Biol._ 7, 291–295 (2010). CAS PubMed Google Scholar * Jenal, M. et al. The poly(A)-binding protein nuclear 1 suppresses alternative cleavage and polyadenylation sites. _Cell_
149, 538–553 (2012). THIS WORK IDENTIFIED THE NUCLEAR POLY(A)-BINDING PROTEIN PABPN1 AS A POTENT SUPPRESSOR OF ALTERNATIVE POLYADENYLATION AND REVEALED THE IMPORTANCE OF APA IN THE PATHOLOGY
OF A HUMAN DISEASE CAUSED BY CHANGES IN PABPN1 LEVELS. CAS PubMed Google Scholar * Martin, G., Gruber, A. R., Keller, W. & Zavolan, M. Genome-wide analysis of pre-mRNA 3′ end
processing reveals a decisive role of human cleavage factor I in the regulation of 3′ UTR length. _Cell Rep._ 1, 753–763 (2012). CAS PubMed Google Scholar * Ruepp, M. D. Schümperli, D.
& Barabino, S.M. mRNA 3′ end processing and more—multiple functions of mammalian cleavage factor I-68. _Wiley Interdiscip_. _Rev. RNA_ 2, 79–91 (2011). CAS PubMed Google Scholar *
Lykke-Andersen, S., Brodersen, D. E. & Jensen, T. H. Origins and activities of the eukaryotic exosome. _J. Cell Sci._ 122, 1487–1494 (2009). CAS PubMed Google Scholar * Butler, J. S.
& Mitchell, P. Rrp6, Rrp47 and cofactors of the nuclear exosome. _Adv. Exp. Med. Biol._ 702, 91–104 (2010). CAS PubMed Google Scholar * Lemay, J. F. et al. The nuclear poly(A)-binding
protein interacts with the exosome to promote synthesis of noncoding small nucleolar RNAs. _Mol. Cell_ 37, 34–45 (2010). CAS PubMed Google Scholar * Lubas, M. et al. Interaction
profiling identifies the human nuclear exosome targeting complex. _Mol. Cell_ 43, 624–637 (2011). CAS PubMed Google Scholar * Shcherbik, N., Wang, M., Lapik, Y. R. Srivastava, L. &
Pestov, D. G. Polyadenylation and degradation of incomplete RNA polymerase I transcripts in mammalian cells. _EMBO Rep._ 11, 106–111 (2010). CAS PubMed PubMed Central Google Scholar *
Guo, T. B. et al. Spermatogenetic expression of RNA-binding motif protein 7, a protein that interacts with splicing factors. _J. Androl._ 24, 204–214 (2003). CAS PubMed Google Scholar *
Nag, A. & Steitz, J. A. Tri-snRNP-associated proteins interact with subunits of the TRAMP and nuclear exosome complexes, linking RNA decay and pre-mRNA splicing. _RNA Biol._ 9, 334–342
(2012). CAS PubMed PubMed Central Google Scholar * Ni, J. Z. et al. Ultraconserved elements are associated with homeostatic control of splicing regulators by alternative splicing and
nonsense-mediated decay. _Genes Dev._ 21, 708–718 (2007). CAS PubMed PubMed Central Google Scholar * Lareau, L. F., Inada, M., Green, R. E., Wengrod, J. C. & Brenner, S. E.
Unproductive splicing of SR genes associated with highly conserved and ultraconserved DNA elements. _Nature_ 446, 926–929 (2007). CAS PubMed Google Scholar * Sureau, A., Gattoni, R.,
Dooghe, Y., Stevenin, J. & Soret, J. SC35 autoregulates its expression by promoting splicing events that destabilize its mRNAs. _EMBO J._ 20, 1785–1796 (2001). CAS PubMed PubMed
Central Google Scholar * Jumaa, H. & Nielsen, P. J. The splicing factor SRp20 modifies splicing of its own mRNA and ASF/SF2 antagonizes this regulation. _EMBO J._ 16, 5077–5085 (1997).
CAS PubMed PubMed Central Google Scholar * Sun, S., Zhang, Z., Sinha, R., Karni, R. & Krainer, A. R. SF2/ASF autoregulation involves multiple layers of post-transcriptional and
translational control. _Nature Struct. Mol. Biol._ 17, 306–312 (2010). CAS Google Scholar * Conti, E. & Izaurralde, E. Nonsense-mediated mRNA decay: molecular insights and mechanistic
variations across species. _Curr. Opin. Cell Biol._ 17, 316–325 (2005). CAS PubMed Google Scholar * Zhang, Z. & Krainer, A. R. Involvement of SR proteins in mRNA surveillance. _Mol.
Cell_ 16, 597–607 (2004). CAS PubMed Google Scholar * Sato, H., Hosoda, N. & Maquat, L. E. Efficiency of the pioneer round of translation affects the cellular site of
nonsense-mediated mRNA decay. _Mol. Cell_ 29, 255–262 (2008). CAS PubMed Google Scholar * Muhlemann, O. & Lykke-Andersen, J. How and where are nonsense mRNAs degraded in mammalian
cells? _RNA Biol._ 7, 28–32 (2010). CAS PubMed PubMed Central Google Scholar * Sun, M. et al. Comparative dynamic transcriptome analysis (cDTA) reveals mutual feedback between mRNA
synthesis and degradation. _Genome Res._ 22, 1350–1359 (2012). CAS PubMed PubMed Central Google Scholar * Dori-Bachash, M., Shema, E. & Tirosh, I. Coupled evolution of transcription
and mRNA degradation. _PLoS Biol._ 9, e1001106 (2011). CAS PubMed PubMed Central Google Scholar * Trcek, T., Larson, D. R., Moldon, A., Query, C. C. & Singer, R. H. Single-molecule
mRNA decay measurements reveal promoter- regulated mRNA stability in yeast. _Cell_ 147, 1484–1497 (2011). USING SINGLE-CELL SINGLE MOLECULE TECHNIQUES, THIS STUDY DEMONSTRATES FOR THE FIRST
TIME A DIRECT LINK BETWEEN NUCLEAR TRANSCRIPTION AND CYTOPLASMIC MRNA STABILITY. CAS PubMed PubMed Central Google Scholar * Bregman, A. et al. Promoter elements regulate cytoplasmic mRNA
decay. _Cell_ 147, 1473–1483 (2011). CAS PubMed Google Scholar * Heym, R. G. & Niessing, D. Principles of mRNA transport in yeast. _Cell. Mol. Life Sci._ 69, 1843–1853 (2012). CAS
PubMed Google Scholar * Shen, Z., St-Denis, A. & Chartrand, P. Cotranscriptional recruitment of She2p by RNA pol II elongation factor Spt4-Spt5/DSIF promotes mRNA localization to the
yeast bud. _Genes Dev._ 24, 1914–1926 (2010). CAS PubMed PubMed Central Google Scholar * Shen, Z., Paquin, N., Forget, A. & Chartrand, P. Nuclear shuttling of She2p couples _ASH1_
mRNA localization to its translational repression by recruiting Loc1p and Puf6p. _Mol. Biol. Cell_ 20, 2265–2275 (2009). CAS PubMed PubMed Central Google Scholar * Long, R. M., Gu, W.,
Lorimer, E., Singer, R. H. & Chartrand, P. She2p is a novel RNA-binding protein that recruits the Myo4p-She3p complex to ASH1 mRNA. _EMBO J._ 19, 6592–6601 (2000). CAS PubMed PubMed
Central Google Scholar * Gu, W., Pan, F., Zhang, H., Bassell, G. J. & Singer, R. H. A predominantly nuclear protein affecting cytoplasmic localization of β-actin mRNA in fibroblasts
and neurons. _J. Cell Biol._ 156, 41–51 (2002). CAS PubMed PubMed Central Google Scholar * Pan, F., Huttelmaier, S., Singer, R. H. & Gu, W. ZBP2 facilitates binding of ZBP1 to
β-actin mRNA during transcription. _Mol. Cell. Biol._ 27, 8340–8351 (2007). CAS PubMed PubMed Central Google Scholar * Hachet, O. & Ephrussi, A. Splicing of oskar RNA in the nucleus
is coupled to its cytoplasmic localization. _Nature_ 428, 959–963 (2004). CAS PubMed Google Scholar * Ghosh, S., Marchand, V., Gaspar, I. & Ephrussi, A. Control of RNP motility and
localization by a splicing-dependent structure in _oskar_ mRNA. _Nature Struct. Mol. Biol._ 19, 441–449 (2012). THIS PAPER PROVIDES A MECHANISTIC LINK BETWEEN NUCLEAR SPLICING AND LOCALIZED
TRANSLATION OF _OSKAR_ MRNA IN THE CYTOPLASM. CAS Google Scholar * Trcek, T. & Singer, R. H. The cytoplasmic fate of an mRNP is determined cotranscriptionally: exception or rule?
_Genes Dev._ 24, 1827–1831 (2010). CAS PubMed PubMed Central Google Scholar * Viphakone, N. et al. TREX exposes the RNA-binding domain of Nxf1 to enable mRNA export. _Nature Commun._ 3,
1006 (2012). Google Scholar * Squires, J. E. et al. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. _Nucleic Acids Res._ 40, 5023–5033 (2012). CAS PubMed
PubMed Central Google Scholar * Dominissini, D. et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. _Nature_ 485, 201–206 (2012). CAS PubMed Google Scholar *
Klug, S. J. & Famulok, M. All you wanted to know about SELEX. _Mol. Biol. Rep._ 20, 97–107 (1994). CAS PubMed Google Scholar * Martin, F. Fifteen years of the yeast three-hybrid
system: RNA–protein interactions under investigation. _Methods_ 58, 367–375 (2012). CAS PubMed Google Scholar * Niranjanakumari, S., Lasda, E., Brazas, R. & Garcia-Blanco, M. A.
Reversible cross-linking combined with immunoprecipitation to study RNA-protein interactions _in vivo_. _Methods_ 26, 182–190 (2002). CAS PubMed Google Scholar * Keene, J. D., Komisarow,
J. M. & Friedersdorf, M. B. RIP-chip: the isolation and identification of mRNAs, microRNAs and protein components of ribonucleoprotein complexes from cell extracts. _Nature Protoc._ 1,
302–307 (2006). CAS Google Scholar * Licatalosi, D. D. et al. HITS-CLIP yields genome-wide insights into brain alternative RNA processing. _Nature_ 456, 464–469 (2008). CAS PubMed PubMed
Central Google Scholar * Hafner, M. et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. _Cell_ 141, 129–141 (2010). CAS PubMed PubMed
Central Google Scholar * Kishore, S. et al. A quantitative analysis of CLIP methods for identifying binding sites of RNA-binding proteins. _Nature Methods_ 8, 559–564 (2011). CAS PubMed
Google Scholar * Kudla, G., Granneman, S., Hahn, D., Beggs, J. D. & Tollervey, D. Cross-linking, ligation, and sequencing of hybrids reveals RNA–RNA interactions in yeast. _Proc. Natl
Acad. Sci. USA_ 108, 10010–10015 (2011). CAS PubMed PubMed Central Google Scholar * Speese, S. D. et al. Nuclear envelope budding enables large ribonucleoprotein particle export during
synaptic Wnt signaling. _Cell_ 149, 832–846 (2012). CAS PubMed PubMed Central Google Scholar * Wu, C. H. et al. NELF and DSIF cause promoter proximal pausing on the hsp70 promoter in
_Drosophila_. _Genes Dev._ 17, 1402–1414 (2003). CAS PubMed PubMed Central Google Scholar * Gruber, J. J. et al. Ars2 links the nuclear cap-binding complex to RNA interference and cell
proliferation. _Cell_ 138, 328–339 (2009). CAS PubMed PubMed Central Google Scholar * Gruber, J. J. et al. Ars2 promotes proper replication-dependent histone mRNA 3′ end formation. _Mol.
Cell_ 45, 87–98 (2012). CAS PubMed PubMed Central Google Scholar * Lahudkar, S. et al. The mRNA cap-binding complex stimulates the formation of pre-initiation complex at the promoter
via its interaction with Mot1p _in vivo_. _Nucleic Acids Res._ 39, 2188–2209 (2011). CAS PubMed Google Scholar * Marzluff, W. F., Wagner, E. J. & Duronio, R. J. Metabolism and
regulation of canonical histone mRNAs: life without a poly(A) tail. _Nature Rev. Genet._ 9, 843–854 (2008). CAS PubMed Google Scholar * Maquat, L. E., Hwang, J., Sato, H. & Tang, Y.
CBP80-promoted mRNP rearrangements during the pioneer round of translation, nonsense-mediated mRNA decay, and thereafter. _Cold Spring Harb. Symp. Quant. Biol._ 75, 127–134 (2010). CAS
PubMed Google Scholar * Visa, N., Izaurralde, E., Ferreira, J., Daneholt, B. & Mattaj, I. W. A nuclear cap-binding complex binds Balbiani ring pre-mRNA cotranscriptionally and
accompanies the ribonucleoprotein particle during nuclear export. _J. Cell Biol._ 133, 5–14 (1996). CAS PubMed Google Scholar * Katahira, J. mRNA export and the TREX complex. _Biochim.
Biophys. Acta_ 1819, 507–513 (2012). CAS PubMed Google Scholar * Hautbergue, G. M. et al. UIF, a new mRNA export adaptor that works together with REF/ALY, requires FACT for recruitment to
mRNA. _Curr. Biol._ 19, 1918–1924 (2009). CAS PubMed PubMed Central Google Scholar * Ruepp, M. D. et al. Mammalian pre-mRNA 3′ end processing factor CF Im68 functions in mRNA export.
_Mol. Biol. Cell_ 20, 5211–5223 (2009). CAS PubMed PubMed Central Google Scholar * Huang, Y., Gattoni, R., Stévenin, J. & Steitz, J. A. S. R. Splicing factors serve as adapter
proteins for TAP-dependent mRNA export. _Mol. Cell_ 11, 837–843 (2003). CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank members of our laboratory, T. Pederson and
F. McNicoll for helpful discussions and comments on the manuscript. Work in our laboratory on mRNPs is supported by funding from the Max Planck Society and the German Research Foundation
(NE-909/3-1 to K.M.N.), and long-term postdoctoral fellowships from the European Molecular Biology Organization (EMBO) and Fonds de recherche en santé du Québec (FRSQ; to M.M.-M.). AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Max Planck Institute of Molecular Cell Biology and Genetics, Pfotenhauerstrasse 108, 01307, Dresden, Germany Michaela Müller-McNicoll & Karla M.
Neugebauer Authors * Michaela Müller-McNicoll View author publications You can also search for this author inPubMed Google Scholar * Karla M. Neugebauer View author publications You can also
search for this author inPubMed Google Scholar CORRESPONDING AUTHORS Correspondence to Michaela Müller-McNicoll or Karla M. Neugebauer. ETHICS DECLARATIONS COMPETING INTERESTS The authors
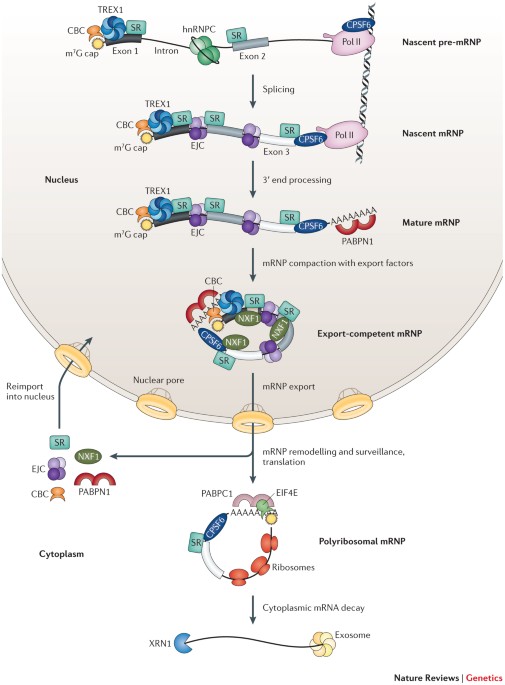
declare no competing financial interests. RELATED LINKS FURTHER INFORMATION Karla M. Neugebauer's homepage POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2
POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 GLOSSARY * Messenger ribonucleoprotein particles (mRNPs). Complexes composed of mature mRNAs bound by various RNA-binding proteins and
associated proteins recruited via protein–protein interactions. The formation of mRNPs allows proper packaging of the mRNA, which is essential for efficient nuclear export. * 5′ end capping
As soon as a nascent transcript emerges from RNA polymerase II during transcription, it is capped at its 5′ end. This 7-methylguanosine cap (m7G cap) protects the mRNA from degradation and
is essential for its translation. * Peptidyl-prolyl-isomerases (PPIs). A group of metabolic enzymes that catalyse the _cis_–_trans_ isomerization of peptide bonds in polypeptide chains. PPIs
have important roles in the folding of newly synthesized proteins but were recently shown also to bind mRNAs. * RNA recognition motif (RRM). One of the most abundant RNA-binding domains in
eukaryotes. * Spliceosome A ribonucleoprotein complex that is responsible for splicing nuclear precursor mRNA (pre-mRNA). It is composed of five small nuclear ribonucleoproteins (snRNPs) and
more than 50 non-snRNP proteins, which recognize and assemble on exon–intron boundaries to catalyse intron removal from the precursor mRNA (pre-mRNA). * Dicer An RNase III family
endonuclease that processes double-stranded RNA and precursor microRNAs into small interfering RNAs and microRNAs, respectively. * Heterogeneous nuclear ribonucleoprotein particles (hnRNPs).
Complexes of newly synthesized precursor mRNA (pre-mRNA) and RNA-binding proteins, known as heterogeneous ribonucleoproteins, which form during transcription in the cell nucleus. The
abundant hnRNP proteins regulate splicing and mark the RNA as immature. After splicing has occurred, the hnRNP proteins mainly remain bound to spliced introns. * Premature cleavage and
polyadenylation (PCA). Misprocessing of precursor mRNAs (pre-mRNA) by the cleavage and polyadenylation machinery. Truncated transcripts arise through the use of cryptic or inappropriate
polyadenylation signals present at the 5′ end or within introns of the pre-mRNA. * R loops Hybrid structures consisting of RNA and DNA in which RNA displaces a DNA strand to hybridize to its
complementary DNA sequence. * Exon junction complex (EJC). A protein complex that is deposited ~24 nucleotides upstream of the exon–exon junctions of newly synthesized, spliced mRNAs. The
EJC contains four core proteins — eukaryotic initiation factor 4AIII (EIF4AIII), Y14, mago nashi homologue (MAGOH) and Barentsz (BTZ) — and several loosely associated proteins. * SR proteins
Evolutionarily conserved RNA-binding proteins with essential functions in precursor mRNA (pre-mRNA) splicing in metazoans. Individual SR proteins have distinct RNA-binding capacities and
are important regulators of alternative splicing, while some also function in post-splicing steps of gene expression. * Balbiani ring Chromosome puffs or large diffused uncoiled regions,
which are the sites of RNA transcription, in the giant polytene chromosomes of _Chironomus tentans_ salivary gland cells. * Exosome A protein complex that has 3′ to 5′ exonuclease activity
(although an endonuclease activity has also been described). Two forms of the exosome have been characterized that differ in their associated cofactors and cellular localization (one is
nuclear and one is cytoplasmic). * Promoter upstream non-coding transcripts (PROMPTs). A recently discovered class of human RNAs. PROMPTs are produced upstream of promoters of active
protein-coding genes. They are mainly nuclear and have poly(A) tails and 5′ cap structures. * Nonsense-mediated mRNA decay (NMD). The process by which mRNAs containing premature termination
codons are destroyed to preclude the production of truncated and potentially deleterious protein products. It is also used in combination with specific alternative splicing events to control
the levels of some proteins. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Müller-McNicoll, M., Neugebauer, K. How cells get the message: dynamic
assembly and function of mRNA–protein complexes. _Nat Rev Genet_ 14, 275–287 (2013). https://doi.org/10.1038/nrg3434 Download citation * Published: 12 March 2013 * Issue Date: April 2013 *
DOI: https://doi.org/10.1038/nrg3434 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative






