- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The advent of precision medicine has prompted profound changes in clinical cancer research, and the rising numbers of new therapeutic agents pose challenges in terms of the most
appropriate trial designs and effects on the drug-approval process. In the past 5 years, some remarkably efficacious drugs have been approved based on evidence from uncontrolled phase I
trials. We challenge the view that the expected benefits from new drugs are generally sufficient to forgo a randomized trial with patients assigned to a control arm (a regimen other than the
experimental treatment). Relying on efficacy results from uncontrolled clinical trials can result in expedited drug approval, but the disadvantages of this practice must be taken into
account. For example, the apparent improvements in outcomes observed in an early single-arm trial of a new therapy might reflect the prognostic nature of the target, rather than a true
treatment effect. Moreover, the predictive role of biomarkers cannot be definitively ascertained without randomly assigning patients to a control arm. We discuss the need for such
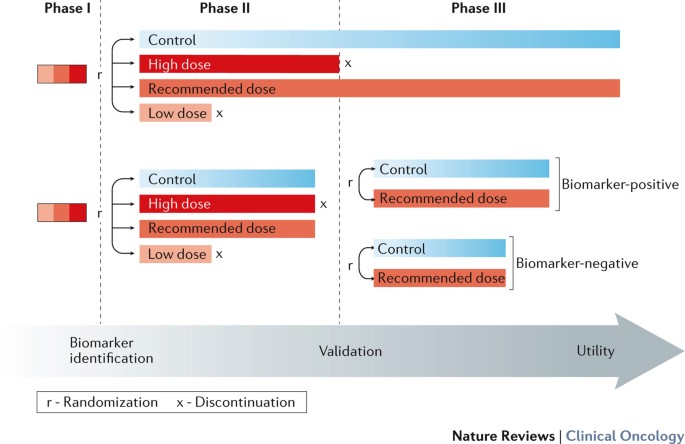
randomization to a true control in all phases of drug development and the role of companion biomarker testing. We propose that an increased use of randomization will facilitate a seamless
transition between phases of drug and/or biomarker development. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS
OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn
more Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to
full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our
FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS CHALLENGES AND OPPORTUNITIES ASSOCIATED WITH THE MD ANDERSON IMPACT2 RANDOMIZED STUDY IN PRECISION ONCOLOGY Article
Open access 27 October 2022 RANDOMISED PHASE 1 CLINICAL TRIALS IN ONCOLOGY Article 10 June 2021 PRECISION MEDICINE: AFFORDING THE SUCCESSES OF SCIENCE Article Open access 04 January 2023
REFERENCES * Masters, G. A. _ et al_. Clinical cancer advances 2015: Annual report on progress against cancer from the American Society of Clinical Oncology. _J. Clin. Oncol._ 33, 786–809
(2015). Article PubMed Google Scholar * US Food & Drug Administration Center for Drug Evaluation & Research. Novel New Drugs 2014 — Summary — January 2015. _FDA_
http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DrugInnovation/UCM430299.pdf (2014). * Malik, S. M. _ et al_. U.S. Food and Drug Administration approval: crizotinib for
treatment of advanced or metastatic non-small cell lung cancer that is anaplastic lymphoma kinase positive. _Clin. Cancer Res._ 20, 2029–2034 (2014). Article CAS PubMed Google Scholar *
Khozin, S. _ et al_. FDA approval: ceritinib for the treatment of metastatic anaplastic lymphoma kinase-positive non-small cell lung cancer. _Clin. Cancer Res._ 21, 2436–2439 (2015). Article
CAS PubMed Google Scholar * Ratain, M. J. & Sargent, D. J. Optimising the design of phase II oncology trials: the importance of randomisation. _Eur. J. Cancer_ 45, 275–280 (2009).
Article PubMed Google Scholar * Hennekens, C. H. & Demets, D. The need for large-scale randomized evidence without undue emphasis on small trials, meta-analyses, or subgroup analyses.
_JAMA_ 302, 2361–2362 (2009). Article CAS PubMed Google Scholar * Hyman, D. M. _ et al_. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. _N. Engl. J. Med._ 373,
726–736 (2015). Article CAS PubMed PubMed Central Google Scholar * Lopez-Chavez, A. _ et al_. Molecular profiling and targeted therapy for advanced thoracic malignancies: a
biomarker-derived, multiarm, multihistology phase II basket trial. _J. Clin. Oncol._ 33, 1000–1007 (2015). Article CAS PubMed PubMed Central Google Scholar * Kim, E. S. _ et al_. The
BATTLE trial: personalizing therapy for lung cancer. _Cancer Discov._ 1, 44–53 (2011). Article CAS PubMed PubMed Central Google Scholar * Mazieres, J. _ et al_. Crizotinib therapy for
advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. _J. Clin. Oncol._ 33, 992–999 (2015). Article CAS PubMed Google Scholar * Kris, M. G. _ et al_.
Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. _JAMA_ 311, 1998–2006 (2014). Article PubMed PubMed Central CAS Google Scholar * American Society
of Clinical Oncology. Targeted Agent and Profiling Utilization Registry (TAPUR). _TAPUR_ http://www.tapur.org/ (2016). * Grove, A. Rethinking clinical trials. _Science_ 333, 1679 (2011).
Article CAS PubMed Google Scholar * Avorn, J. & Kesselheim, A. S. The 21st Century Cures Act — will it take us back in time? _N. Engl. J. Med._ 372, 2473–2475 (2015). Article CAS
PubMed Google Scholar * Buyse, M., Sargent, D. J., Grothey, A., Matheson, A. & de Gramont, A. Biomarkers and surrogate end points — the challenge of statistical validation. _Nat. Rev.
Clin. Oncol._ 7, 309–317 (2010). Article PubMed Google Scholar * Dahlberg, S. E., Shapiro, G. I., Clark, J. W. & Johnson, B. E. Evaluation of statistical designs in phase I expansion
cohorts: the Dana-Farber/Harvard Cancer Center experience. _J. Natl Cancer Inst._ 106, dju163 (2014). Article PubMed Google Scholar * Manji, A. _ et al_. Evolution of clinical trial
design in early drug development: systematic review of expansion cohort use in single-agent phase I cancer trials. _J. Clin. Oncol._ 31, 4260–4267 (2013). Article CAS PubMed Google
Scholar * Rogatko, A. _ et al_. Translation of innovative designs into phase I trials. _J. Clin. Oncol._ 25, 4982–4986 (2007). Article PubMed Google Scholar * Le Tourneau, C., Lee, J. J.
& Siu, L. L. Dose escalation methods in phase I cancer clinical trials. _J. Natl Cancer Inst._ 101, 708–720 (2009). Article CAS PubMed PubMed Central Google Scholar * Riviere, M.
K., Le Tourneau, C., Paoletti, X., Dubois, F. & Zohar, S. Designs of drug-combination phase I trials in oncology: a systematic review of the literature. _Ann. Oncol._ 26, 669–674 (2015).
Article PubMed Google Scholar * Paoletti, X., Ezzalfani, M. & Le Tourneau, C. Statistical controversies in clinical research: requiem for the 3 + 3 design for phase I trials. _Ann.
Oncol._ 26, 1808–1812 (2015). Article CAS PubMed PubMed Central Google Scholar * Weber, J. S. _ et al_. American Society of Clinical Oncology policy statement update: the critical role
of phase I trials in cancer research and treatment. _J. Clin. Oncol._ 33, 278–284 (2015). Article PubMed Google Scholar * Lee, J. J. & Feng, L. Randomized phase II designs in cancer
clinical trials: current status and future directions. _J. Clin. Oncol._ 23, 4450–4457 (2005). Article PubMed Google Scholar * Sleijfer, S., Bogaerts, J. & Siu, L. L. Designing
transformative clinical trials in the cancer genome era. _J. Clin. Oncol._ 31, 1834–1841 (2013). Article PubMed Google Scholar * Sharma, M. R., Stadler, W. M. & Ratain, M. J.
Randomized phase II trials: a long-term investment with promising returns. _J. Natl Cancer Inst._ 103, 1093–1100 (2011). Article CAS PubMed PubMed Central Google Scholar * Mandrekar, S.
J. & Sargent, D. J. Clinical trial designs for predictive biomarker validation: theoretical considerations and practical challenges. _J. Clin. Oncol._ 27, 4027–4034 (2009). Article
PubMed PubMed Central Google Scholar * Buyse, M. & Michiels, S. Omics-based clinical trial designs. _Curr. Opin. Oncol._ 25, 289–295 (2013). Article PubMed Google Scholar *
McLaughlin, P. _ et al_. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. _J. Clin. Oncol._
16, 2825–2833 (1998). Article CAS PubMed Google Scholar * Druker, B. J. _ et al_. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia.
_N. Engl. J. Med._ 344, 1031–1037 (2001). Article CAS PubMed Google Scholar * Mok, T. S. _ et al_. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. _N. Engl. J. Med._
361, 947–957 (2009). Article CAS PubMed Google Scholar * Herbst, R. S. _ et al_. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase
III trial — INTACT 2. _J. Clin. Oncol._ 22, 785–794 (2004). Article CAS PubMed Google Scholar * Williams, R. Discontinued in 2013: oncology drugs. _Expert Opin. Investig. Drugs_ 24,
95–110 (2015). Article CAS PubMed Google Scholar * Tsimberidou, A. M. _ et al_. Personalized medicine for patients with advanced cancer in the phase I program at MD Anderson: validation
and landmark analyses. _Clin. Cancer Res._ 20, 4827–4836 (2014). Article CAS PubMed PubMed Central Google Scholar * Schwaederle, M. _ et al_. Impact of precision medicine in diverse
cancers: a meta-analysis of phase II clinical trials. _J. Clin. Oncol._ 33, 3817–3825 (2015). Article CAS PubMed PubMed Central Google Scholar * Fontes Jardim, D. L. _ et al_. Impact of
a biomarker-based strategy on oncology drug development: a meta-analysis of clinical trials leading to FDA approval. _J. Natl Cancer Inst._ 107, djv253 (2015). Article PubMed PubMed
Central Google Scholar * Schwaederle, M. _ et al_. Association of biomarker-based treatment strategies with response rates and progression-free survival in refractory malignant neoplasms:
a meta-analysis. _JAMA Oncol._ 2, 1452–1459 (2016). Article PubMed Google Scholar * Dienstmann, R. _ et al_. Molecular profiling of patients with colorectal cancer and matched targeted
therapy in phase I clinical trials. _Mol. Cancer Ther._ 11, 2062–2071 (2012). Article CAS PubMed Google Scholar * Le Tourneau, C. _ et al_. Molecularly targeted therapy based on tumour
molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. _Lancet Oncol._ 16, 1324–1334
(2015). Article CAS PubMed Google Scholar * Catenacci, D. V. Expansion platform type II: testing a treatment strategy. _Lancet Oncol._ 16, 1276–1278 (2015). Article PubMed PubMed
Central Google Scholar * Le Tourneau, C. & Kurzrock, R. Targeted therapies: what have we learned from SHIVA? _Nat. Rev. Clin. Oncol._ 13, 719–720 (2016). Article CAS PubMed Google
Scholar * Gerlinger, M. _ et al_. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. _N. Engl. J. Med._ 366, 883–892 (2012). Article CAS PubMed PubMed
Central Google Scholar * Liu, X. _ et al_. Iniparib nonselectively modifies cysteine-containing proteins in tumor cells and is not a _bona fide_ PARP inhibitor. _Clin. Cancer Res._ 18,
510–523 (2012). Article CAS PubMed Google Scholar * Eberhard, D. A. _ et al_. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in
patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. _J. Clin. Oncol._ 23, 5900–5909 (2005). Article CAS PubMed Google Scholar *
Schmoor, C. & Schumacher, M. Methodological arguments for the necessity of randomized trials in high-dose chemotherapy for breast cancer. _Breast Cancer Res. Treat._ 54, 31–38 (1999).
Article CAS PubMed Google Scholar * Tannock, I. F. Some problems related to the design and analysis of clinical trials. _Int. J. Radiat. Oncol. Biol. Phys._ 22, 881–885 (1992). Article
CAS PubMed Google Scholar * Chabner, B. A. Early accelerated approval for highly targeted cancer drugs. _N. Engl. J. Med._ 364, 1087–1089 (2011). Article CAS PubMed Google Scholar *
Kummar, S. _ et al_. Application of molecular profiling in clinical trials for advanced metastatic cancers. _J. Natl Cancer Inst._ 107, djv003 (2015). Article PubMed PubMed Central CAS
Google Scholar * Kurzrock, R. & Stewart, D. J. Equipoise abandoned? Randomization and clinical trials. _Ann. Oncol._ 24, 2471–2474 (2013). Article CAS PubMed Google Scholar *
Djulbegovic, B., Hozo, I. & Ioannidis, J. P. Improving the drug development process: more not less randomized trials. _JAMA_ 311, 355–356 (2014). Article CAS PubMed Google Scholar *
Gehan, E. A. & Freireich, E. J. Non-randomized controls in cancer clinical trials. _N. Engl. J. Med._ 290, 198–203 (1974). Article CAS PubMed Google Scholar * Sanwald-Ducray, P.,
Liogier D'ardhuy, X., Jamois, C. & Banken, L. Pharmacokinetics, pharmacodynamics, and tolerability of aleglitazar in patients with type 2 diabetes: results from a randomized,
placebo-controlled clinical study. _Clin. Pharmacol. Ther._ 88, 197–203 (2010). Article CAS PubMed Google Scholar * Perlstein, I., Bolognese, J. A., Krishna, R. & Wagner, J. A.
Evaluation of agile designs in first-in-human (FIH) trials — a simulation study. _AAPS J._ 11, 653–663 (2009). Article CAS PubMed PubMed Central Google Scholar * Mathijssen, R. H.,
Sparreboom, A. & Verweij, J. Determining the optimal dose in the development of anticancer agents. _Nat. Rev. Clin. Oncol._ 11, 272–281 (2014). Article CAS PubMed Google Scholar *
Postel-Vinay, S. _ et al_. Towards new methods for the determination of dose limiting toxicities and the assessment of the recommended dose for further studies of molecularly targeted agents
— Dose-Limiting Toxicity and Toxicity Assessment Recommendation Group for Early Trials of Targeted therapies, an European Organisation for Research and Treatment of Cancer-led study. _Eur.
J. Cancer_ 50, 2040–2049 (2014). Article PubMed Google Scholar * Jain, R. K. _ et al_. Phase I oncology studies: evidence that in the era of targeted therapies patients on lower doses do
not fare worse. _Clin. Cancer Res._ 16, 1289–1297 (2010). Article CAS PubMed PubMed Central Google Scholar * Sachs, J. R., Mayawala, K., Gadamsetty, S., Kang, S. P. & de Alwis, D.
P. Optimal dosing for targeted therapies in oncology: drug development cases leading by example. _Clin. Cancer Res._ 22, 1318–1324 (2016). Article CAS PubMed Google Scholar * Agrawal, M.
& Emanuel, E. J. Ethics of phase 1 oncology studies: reexamining the arguments and data. _JAMA_ 290, 1075–1082 (2003). Article PubMed Google Scholar * Kodish, E., Stocking, C.,
Ratain, M. J., Kohrman, A. & Siegler, M. Ethical issues in phase I oncology research: a comparison of investigators and institutional review board chairpersons. _J. Clin. Oncol._ 10,
1810–1816 (1992). Article CAS PubMed Google Scholar * Roberts, T. G. Jr _ et al_. Trends in the risks and benefits to patients with cancer participating in phase 1 clinical trials.
_JAMA_ 292, 2130–2140 (2004). Article CAS PubMed Google Scholar * Flaherty, K. T. _ et al_. Inhibition of mutated, activated BRAF in metastatic melanoma. _N. Engl. J. Med._ 363, 809–819
(2010). Article CAS PubMed PubMed Central Google Scholar * Janne, P. A. _ et al_. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. _N. Engl. J. Med._ 372, 1689–1699
(2015). Article PubMed Google Scholar * Cortes, J. E. _ et al_. Ponatinib in refractory Philadelphia chromosome-positive leukemias. _N. Engl. J. Med._ 367, 2075–2088 (2012). Article CAS
PubMed PubMed Central Google Scholar * Horstmann, E. _ et al_. Risks and benefits of phase 1 oncology trials, 1991 through 2002. _N. Engl. J. Med._ 352, 895–904 (2005). Article CAS
PubMed Google Scholar * Estey, E. _ et al_. Therapeutic response in phase I trials of antineoplastic agents. _Cancer Treat. Rep._ 70, 1105–1115 (1986). CAS PubMed Google Scholar *
Robert, C. _ et al_. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial.
_Lancet_ 384, 1109–1117 (2014). Article CAS PubMed Google Scholar * Theoret, M. R. _ et al_. Expansion cohorts in first-in- human solid tumor oncology trials. _Clin. Cancer Res._ 21,
4545–4551 (2015). Article PubMed Google Scholar * Chalmers, T. C. Randomization of the first patient. _Med. Clin. North Am._ 59, 1035–1038 (1975). Article CAS PubMed Google Scholar *
Johnson, J. R., Williams, G. & Pazdur, R. End points and United States Food and Drug Administration approval of oncology drugs. _J. Clin. Oncol._ 21, 1404–1411 (2003). Article PubMed
Google Scholar * Simon, R., Wittes, R. E. & Ellenberg, S. S. Randomized phase II clinical trials. _Cancer Treat. Rep._ 69, 1375–1381 (1985). CAS PubMed Google Scholar * Saad, E. D. _
et al_. Formal statistical testing and inference in randomized phase II trials in medical oncology. _Am. J. Clin. Oncol._ 36, 143–145 (2013). Article PubMed Google Scholar * Tang, H. _
et al_. Comparison of error rates in single-arm versus randomized phase II cancer clinical trials. _J. Clin. Oncol._ 28, 1936–1941 (2010). Article PubMed PubMed Central Google Scholar *
Jardim, D. L., Groves, E. S., Breitfeld, P. P. & Kurzrock, R. Factors associated with failure of oncology drugs in late-stage clinical development: a systematic review. _Cancer Treat.
Rev._ 52, 12–21 (2016). Article PubMed Google Scholar * Hey, S. P. & Kimmelman, J. Are outcome-adaptive allocation trials ethical? _Clin. Trials_ 12, 102–106 (2015). Article PubMed
PubMed Central Google Scholar * Buyse, M. Commentary on Hey and Kimmelman. _Clin. Trials_ 12, 119–121 (2015). Article PubMed Google Scholar * Harrington, D. & Parmigiani, G. I-SPY 2
— a glimpse of the future of phase 2 drug development? _N. Engl. J. Med._ 375, 7–9 (2016). Article PubMed Google Scholar * Park, J. W. _ et al_. Adaptive randomization of neratinib in
early breast cancer. _N. Engl. J. Med._ 375, 11–22 (2016). Article CAS PubMed PubMed Central Google Scholar * Korn, E. L. & Freidlin, B. Outcome — adaptive randomization: is it
useful? _J. Clin. Oncol._ 29, 771–776 (2011). Article PubMed Google Scholar * Saxman, S. B. Ethical considerations for outcome-adaptive trial designs: a clinical researcher's
perspective. _Bioethics_ 29, 59–65 (2015). Article PubMed Google Scholar * Simon, R. _ et al_. The role of nonrandomized trials in the evaluation of oncology drugs. _Clin. Pharmacol.
Ther._ 97, 502–507 (2015). Article CAS PubMed Google Scholar * Boyd, N., Dancey, J. E., Gilks, C. B. & Huntsman, D. G. Rare cancers: a sea of opportunity. _Lancet Oncol._ 17, e52–e61
(2016). Article PubMed Google Scholar * Bayar, M. A., Le Teuff, G., Michiels, S., Sargent, D. J. & Le Deley, M. C. New insights into the evaluation of randomized controlled trials
for rare diseases over a long-term research horizon: a simulation study. _Stat. Med._ 35, 3245–3258 (2016). Article PubMed Google Scholar * Gan, H. K. _ et al_. Randomized phase II
trials: inevitable or inadvisable? _J. Clin. Oncol._ 28, 2641–2647 (2010). Article PubMed Google Scholar * Ellis, M. J. _ et al_. Fulvestrant 500 mg versus anastrozole 1 mg for the
first-line treatment of advanced breast cancer: overall survival analysis from the phase II FIRST Study. _J. Clin. Oncol._ 33, 3781–3787 (2015). Article CAS PubMed PubMed Central Google
Scholar * Sehn, L. H. _ et al_. Randomized phase II trial comparing obinutuzumab (GA101) with rituximab in patients with relapsed CD20+ indolent B-cell non-Hodgkin lymphoma: final analysis
of the GAUSS Study. _J. Clin. Oncol._ 33, 3467–3474 (2015). Article CAS PubMed PubMed Central Google Scholar * Rosell, R. _ et al_. Erlotinib versus standard chemotherapy as first-line
treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. _Lancet Oncol._ 13, 239–246
(2012). Article CAS PubMed Google Scholar * Zhou, C. _ et al_. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung
cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. _Lancet Oncol._ 12, 735–742 (2011). Article CAS PubMed Google Scholar * Buyse, M. Limitations of
adaptive clinical trials. _Am. Soc. Clin. Oncol. Educ. Book_ 2012, 133–137 (2012). Article Google Scholar * Fischl, M. A. _ et al_. The efficacy of azidothymidine (AZT) in the treatment of
patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. _N. Engl. J. Med._ 317, 185–191 (1987). Article CAS PubMed Google Scholar Download references
ACKNOWLEDGEMENTS We thank the three reviewers who anonymously contributed to our paper with their insightful and constructive critique. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Everardo
D. Saad is at the International Drug Development Institute (IDDI), Avenue Provinciale 30, 1340 Ottignies-Louvain-la-Neuve, Belgium., Everardo D. Saad * Xavier Paoletti is at the Institut
Gustave Roussy, 114 Rue Edouard Vaillant, 94800 Villejuif, France., Xavier Paoletti * Tomasz Burzykowski is at the IDDI, Avenue Provinciale 30, 1340 Ottignies-Louvain-la-Neuve, Belgium,
Tomasz Burzykowski * at the Interuniversity Institute for Biostatistics and Statistical Bioinformatics (I-BioStat), Hasselt University, Martelarenlaan 42, 3500 Hasselt, Belgium., Tomasz
Burzykowski * Marc Buyse is at the I-BioStat, Hasselt University, Martelarenlaan 42, 3500 Hasselt, Belgium, Marc Buyse * at the IDDI, 185 Alewife Brook Parkway, Suite 410, Cambridge,
Massachusetts 02138, USA., Marc Buyse Authors * Everardo D. Saad View author publications You can also search for this author inPubMed Google Scholar * Xavier Paoletti View author
publications You can also search for this author inPubMed Google Scholar * Tomasz Burzykowski View author publications You can also search for this author inPubMed Google Scholar * Marc
Buyse View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS All authors researched data for the article and contributed to discussions of the
article's content. E.D.S. and M.B. wrote, reviewed, and edited the manuscript before submission. CORRESPONDING AUTHOR Correspondence to Everardo D. Saad. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing financial interests. POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR TABLE 1 RIGHTS AND PERMISSIONS Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE Saad, E., Paoletti, X., Burzykowski, T. _et al._ Precision medicine needs randomized clinical trials. _Nat Rev Clin Oncol_ 14, 317–323 (2017).
https://doi.org/10.1038/nrclinonc.2017.8 Download citation * Published: 07 February 2017 * Issue Date: May 2017 * DOI: https://doi.org/10.1038/nrclinonc.2017.8 SHARE THIS ARTICLE Anyone you
share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the
Springer Nature SharedIt content-sharing initiative