- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Heart failure is a pressing worldwide public-health problem with millions of patients having worsening heart failure. Despite all the available therapies, the condition carries a
very poor prognosis. Existing therapies provide symptomatic and clinical benefit, but do not fully address molecular abnormalities that occur in cardiomyocytes. This shortcoming is
particularly important given that most patients with heart failure have viable dysfunctional myocardium, in which an improvement or normalization of function might be possible. Although the
pathophysiology of heart failure is complex, mitochondrial dysfunction seems to be an important target for therapy to improve cardiac function directly. Mitochondrial abnormalities include
impaired mitochondrial electron transport chain activity, increased formation of reactive oxygen species, shifted metabolic substrate utilization, aberrant mitochondrial dynamics, and
altered ion homeostasis. In this Consensus Statement, insights into the mechanisms of mitochondrial dysfunction in heart failure are presented, along with an overview of emerging treatments
with the potential to improve the function of the failing heart by targeting mitochondria. SIMILAR CONTENT BEING VIEWED BY OTHERS MITOCHONDRIAL CA2+ REGULATION IN THE ETIOLOGY OF HEART
FAILURE: PHYSIOLOGICAL AND PATHOPHYSIOLOGICAL IMPLICATIONS Article 21 July 2020 MITOCHONDRIAL QUALITY CONTROL IN CARDIOMYOCYTES: SAFEGUARDING THE HEART AGAINST DISEASE AND AGEING Article 20
March 2025 MITOPHAGY IN ISCHEMIC HEART DISEASE: MOLECULAR MECHANISMS AND CLINICAL MANAGEMENT Article Open access 30 December 2024 MAIN Heart failure (HF) is associated with substantial
clinical burden and economic costs worldwide. The disease is particularly prevalent in elderly individuals, in whom the incidence and associated costs are projected to double over the next
20 years1,2. Economic costs associated with the management of patients with HF is estimated at >US$30 billion annually in the USA alone, and accounts for roughly 2–3% of total health-care
spending globally3,4. Despite these enormous costs, mortality from HF remains high. Death from HF within 5 years of diagnosis is common despite current optimal medical therapy. Mortality
and rehospitalization within 60–90 days after discharge from hospital can be as high as 15% and 35%, respectively5. These event rates have largely not changed over the past 15 years, despite
implementation of evidence-based therapy5. HF rehospitalization rates also remain high, with care typically focused on symptomatic relief. Patients with HF are often designated as having
either reduced ejection fraction (HFrEF), or preserved ejection fraction (HFpEF). Patients with HFpEF also have poor prognosis after the first diagnosis6. Regardless of the HF aetiology,
novel treatments that improve intrinsic cardiac function remain elusive. Advances in the treatment of ischaemic and valvular heart disease have clearly improved patient survival. The
residual cardiac dysfunction and associated comorbidities, however, have led, in the long-term, to the development of HF with attendant poor quality of life. Commonly prescribed HF
medications, although beneficial in promoting some symptom relief, often do not fully address the underlying causes of progressive left ventricular dysfunction7. Most standard-of-care
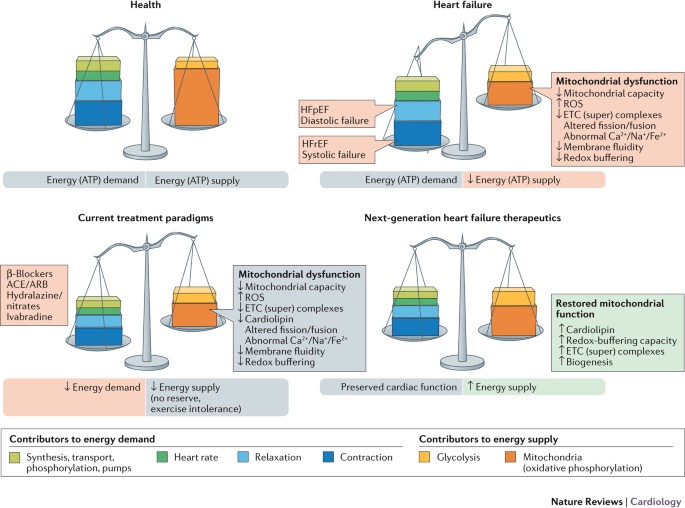
pharmacological approaches to HF act by reducing workload on the failing heart and, in doing so, attempt to rebalance energy supply and energy demand, albeit to a lower level (Fig. 1).
Hallmarks of current therapies include modulation of neurohormonal abnormalities, unloading the heart (that is, vasodilatation), and/or reducing the heart rate — all important determinants
of reducing myocardial oxygen consumption8. β-Blockers, ivabradine, and antagonism of the renin–angiotensin–aldosterone system all act in concert to reduce myocardial energy requirements and
attenuate or prevent further adverse cardiac remodelling. Although these therapies have improved survival in patients with chronic ambulatory HFrEF over the past 2–3 decades, death and poor
quality of life continue to adversely affect this ever-increasing patient population. This unmet need is probably not going to be met by drugs that modulate neurohormonal abnormalities and
lower heart rates, because further intervention along these axes is likely to be counterproductive as hypotension and bradycardia become limiting factors. The search for more effective and
complementary therapy for this patient population must be focused on improving the intrinsic function of the viable, but dysfunctional, cardiac unit — the cardiomyocytes3,9. The novel
therapy must be haemodynamically neutral (no decrease in blood pressure or heart rate) and must target the myocardium as the centrepiece of the therapeutic intervention10. The vast majority
of phase III trials in patients with HF conducted in the past decade have been negative, arguably for the same reasons discussed above11,12. Furthermore, a relative underinvestment in
cardiovascular drug development, as well as strategic abandonment by pharmaceutical companies of new therapies for which the risks are perceived to be higher than the rewards, have also
contributed to slow development of drugs for HF13. Moreover, the development of effective therapies for HFpEF is imperative to treat this patient population, but the variability in HFpEF
phenotypes (such as age, and the presence of diabetes mellitus or hypertension), and the difficulty in establishing reliable preclinical models of HFpEF, also hinder progress. Despite these
obstacles, ample opportunity exists to improve HF treatments, provided the focus is directed towards cardiomyocytes and their intrinsic function. A roundtable meeting was held in Stresa,
Italy on 23 October 2015 to discuss the multifaceted problem of insufficient energy production in HF, and the role it has in progressive left ventricular dysfunction. This meeting was
attended by academics, clinicians, and representatives from the pharmaceutical industry. The meeting focused on mitochondrial dysfunction as the source of energy deprivation in HF, and how
correction of mitochondrial dysfunction using emerging novel therapies might lead to functional improvement of the HF phenotype. This Consensus Statement summarizes the findings from that
roundtable discussion. BIOENERGETICS OF THE BEATING HEART Aristotle considered the heart to be the body's furnace, radiating energy in the form of heat14. Given the astounding energetic
cost of cardiac function, this concept is not so far from the truth. Humans produce and consume roughly their body weight in ATP (about 65 kg) every single day15. The heart accounts for
only ∼0.5% of body weight, but is responsible for roughly 8% of ATP consumption. This high energy flux is dynamic: the heart stores only enough energy to support pumping for a few heart
beats, turning over the entire metabolite pool approximately every 10 s even at resting heart rates16. As the most metabolically active organ in the body, the heart possesses the highest
content of mitochondria of any tissue. Mitochondria comprise 25–30% of cell volume across mammalian species17,18, with only the myofilaments being more densely packed within cardiac
myocytes. The high mitochondrial content of cardiomyocytes is needed to meet the enormous energy requirement for contraction and relaxation (which is also an active process). About 90% of
cellular ATP is utilized to support the contraction–relaxation cycle within the myocardium19. ATP-dependent release of actin from myosin is required for both contraction (as myosin heads
cycle through cross-bridges with actin) and relaxation. Cellular sequestration of calcium back into the sarcoplasmic reticulum during diastole also requires a tremendous amount of ATP. Cells
sustain the energy requirements necessary to support cardiac function through remarkable metabolic supply–demand matching20,21 (Fig. 1). Bioenergetic homeostasis is accomplished almost
exclusively through an 'energy grid' comprised of a mitochondrial network and their associated phosphate-transfer couples. Cardiac mitochondria must operate at high efficiency
levels to respond instantaneously to the energetic needs of contractile units, a demand that is ever-changing and necessitated by the body's dynamic requirements for oxygen-bearing
blood. Myocardial energy requirements are more pronounced during physical activity, when demands for energy increase to maintain cardiac function commensurate with the needs of the body.
However, other mitochondrial abnormalities besides energy deprivation during physical activity can contribute to the pathologies seen in patients with HF. Mitochondrial abnormalities in HF
are not only a question of reduced capacity to generate ATP (even though that capacity is reduced at rest in HF compared with resting normal), but can also be directly linked to
cardiomyocyte injury and death and, therefore, to disease progression. Abnormal mitochondria are a major source of reactive oxygen species (ROS) production, which can induce cellular damage.
Abnormal mitochondria can promote programmed cell death through the release of cytochrome _c_ into the cytosolic compartment and activation of caspases. Therefore, mitochondria directly
influence ongoing cell injury and death. Mitochondrial abnormalities have also been implicated in aberrant cellular calcium homeostasis, vascular smooth muscle pathology, myofibrillar
disruption, and altered cell differentiation, all important issues in cardiovascular disease, including HF. MITOCHONDRIA IN CARDIOMYOCYTES Mitochondria are primarily located within
subsarcolemmal, perinuclear, and intrafibrillar regions of the cardiomyocyte. Although they are symbiotic partners with the other cellular compartments, mitochondria are in many ways
discrete entities. Mitochondrial dynamics in the form of fission, fusion, and autophagy are highly regulated processes that are essential for energy production and structural integrity of
the organelles22,23,24,25,26,27,28,29. Altered mitochondrial biogenesis, fragmentation, and hyperplasia have been observed in studies of human30 and animal models31,32 of HF. These effects
seem to be caused by altered expression of proteins that regulate mitochondrial dynamics33. As many of these factors are 'master regulators' of mitochondrial metabolism, these
changes might be directly related to the decreased capacity to oxidize fatty acid substrates often seen in HF34,35. Mitochondria have their own DNA (mtDNA) and a genetic code that is
distinct from the host-cell nuclear DNA. mtDNA is circular in shape, analogous to DNA found in lower organisms, and a primitive fingerprint leftover from bacterial origin. Evolutionary
selection pressures have led to mitochondria 'outsourcing' almost all their protein-making needs to their cellular hosts. The overwhelming majority (>99%) of mitochondrial
proteins come from nuclear-encoded DNA. These proteins are synthesized via cellular protein synthesis machinery, and are actively imported into mitochondria through mitochondrial membrane
transporters36. mtDNA encodes 13 protein subunits found within three of the electron transport protein complexes, and a handful of ribosomal and transfer RNAs37. These proteins are made in
specialized ribosomes or 'mitoribosomes', which are physically attached to the mitochondrial inner membrane38. Many inherited familial cardiomyopathies (both adult and paediatric)
are associated with mtDNA mutations39. In humans, mitochondria are maternally inherited40, owing to high mitochondrial density in the egg and the active degradation of mitochondria in the
sperm during fertilization41. The proximity of mtDNA to sites of mitochondrial ROS generation, poor repair mechanisms, and a lack of protective histones combine to make mtDNA particularly
susceptible to oxidative injury and mutation. Mitochondrial genetics contribute to cardiomyopathies by expressing mutant proteins that influence energy homeostasis. With 1,000–10,000 genes
per mitochondria (polyploidy), mitochondrial genetics operate on population-based (instead of Mendelian) principles37. Mutated mtDNA is found alongside nonmutated copies, leading to
mitochondrial 'heteroplasmy'. The extent of heteroplasmy in mutated mtDNA influences the susceptibility to inherited mitochondrial disease42. Mutated mtDNA can be found in 1 in 200
individuals, a frequency that is 20-fold higher than the incidence of mitochondrial disease. This mismatch indicates that healthy individuals often harbour mutated mtDNA that has no
observable phenotypic consequences until a certain mutation threshold is reached37. Although very early in preclinical development, various innovative approaches to reduce the extent of
heteroplasmy using genome editing might ultimately lead to effective therapy for HF caused by genetic mitochondrial disease43,44,45. Given that mitochondrial abnormalities, such as increased
ROS production, altered mitochondrial energetics, and impaired mitochondrial ion homeostasis, are observed in genetic mitochondrial diseases as well as HF, innovative approaches that target
mitochondrial dysfunction might share efficacy across these diseases. HEART FAILURE IS A BIOENERGETIC DISEASE The 'myocardial power grid' consists of mitochondrial ATP supply that
transfers energy throughout the cell along intracellular phosphotransfer buffering systems (Fig. 2). Mitochondria utilize carbon sources from food substrates, which are catabolized and
passed through the Krebs cycle and are then channelled through a series of redox reactions along the inner mitochondrial membrane. The oxidation of these substrates creates a proton
electrochemical gradient, predominantly in the form of mitochondrial membrane potential (ΔΨm)46. Protons that re-enter the mitochondrial matrix through complex V (mitochondrial ATP synthase)
liberate energy that phosphorylates ADP, regenerating ATP. Newly synthesized ATP is rapidly transferred out of mitochondria and energy is subsequently distributed throughout the cell via
reversible phosphate exchange networks, primarily catalysed by creatine kinase and adenylate kinase-associated reactions16,47. The evidence that HF involves impaired cellular energy
production and transfer is considerable (Table 1). Among studies that have directly examined energetics in human HF, all but three noted some form of bioenergetic impairment in the failing
heart. This decrement in bioenergetics is reflected by a decrease in cellular ATP, phosphocreatine (PCr), or the PCr/ATP ratio. Impaired bioenergetics affect patients with HFrEF and those
with HFpEF (Table 1). Although it is difficult to tell from the heterogeneous patient population included in Table 1, the progression to HF is likely to be associated with a gradual decline
in bioenergetic reserve capacity that ultimately reaches a critical threshold, after which endogenous mechanisms can no longer compensate for faltering energy supply48. Attempts to improve
bioenergetics in HF tend to focus on mitochondrial energy production as a target, because direct augmentation of myocardial creatine with oral creatine supplementation is thwarted by a
decreased capacity to transport creatine into the failing cardiomyocytes49. Skeletal muscles also show mitochondrial dysfunction in HF, contributing to the exercise intolerance that
characterizes the HF state50. Abnormal mitochondrial function has also been reported in patients with renal insufficiency51, and in patients with insulin resistance52. Given that patients
with HF often manifest both renal insufficiency and insulin resistance, treating mitochondrial dysfunction in HF derives benefits that go beyond improving cardiac function (Fig. 3). Several
interventions are currently being tested in clinical trials to stimulate mitochondrial biogenesis in HF. These include epicatechin and resveratrol, which are naturally-occurring polyphenols
found in foods such as red wine, green tea, and dark chocolate. Preclinical HF models suggest that these molecules are biologically active53,54,55, and some success in improving cardiac
function has been reported in small trials of patients with myocardial infarction56. Larger trials in patients with HF are required. MITOCHONDRIAL SUBSTRATE SELECTIVITY Substrate utilization
in the failing heart has been extensively reviewed previously57,58,59,60. Overall, altered substrate metabolism seems to be centrally involved in HF, although the direction of the metabolic
alterations is complex and is likely to depend on the particular stage of HF progression and differences in the availability of substrate (whether the heart is in a 'fed' or
'fasted' state)58,59. The heart utilizes different substrates simultaneously to produce energy. Mitochondrial fatty acid oxidation (FAO) is the predominate substrate used in the
healthy adult human heart, being responsible for 60–80% of cardiac ATP production, followed by lesser contributions from glucose, lactate, and ketone bodies61. However, the heart can shift
the relative contribution of these substrates in an effort to adapt to varying physiological conditions. Under conditions of low oxygen content, such as ischaemia and HF, ATP content is
thought to decrease by as much as 40%3. In HF, fatty acid oxidation and the oxidative capacity of the mitochondria decline, and can no longer maintain sufficient levels of ATP, especially
during conditions of increased cardiac workload such as exercise. The failing heart shifts its predominant fuel source from mitochondrial FAO toward glycolytic pathways. This switch is most
apparent in late and end-stage HF57, and is 30% more energetically efficient in the failing heart, because more ATP is produced per mole of oxygen during carbohydrate oxidation62. Numerous
studies investigating FAO, glucose oxidation, and (to a lesser extent) ketone body oxidation have aimed to establish a metabolic phenotype, underlying molecular mechanisms, and potential
therapeutic targets of the failing heart. The reduction in fatty acid uptake and FAO that occurs during HF might be owing to dysregulated molecular mechanisms responsible for fatty acid
metabolism. For example, the level of peroxisome proliferator-activated receptor-α (PPARα), a transcription factor highly expressed in the heart and responsible for fatty acid transport into
the mitochondria and peroxisomes, has been reported to be downregulated in both animal models and humans with HF63,64. Similarly, tissue from animals and humans with HF has reduced activity
of the transcription factor responsible for mitochondrial biogenesis, PPAR-γ co-activator (PGC)-1α64,65. Because these transcription factors have a critical role in the regulation of
cardiac mitochondrial energy production, these data suggest that decreased PPARα and PGC-1α activity might be an important precursor leading to impaired FAO during HF. Therefore, further
inhibition of FAO to increase glycolytic flux via PPARα and/or PGC-1α is a plausible therapeutic target. Small-molecule regulators of PGC-1α are needed, and animal models overexpressing the
transcription factor are inherently problematic, ostensibly owing to increased mitochondrial biogenesis-induced cardiomyopathy66. Similarly, PPARα antagonists in animal models of HF have
yielded inconclusive data67, whereas clinical PPARα ligands are reportedly safe, but their efficacy in a HF population is currently unknown61. Although the safety of PPARα ligands is
promising, further evidence demonstrating their efficacy in both animal models and humans with HF is needed. Levels of circulating free fatty acids might be higher in the failing heart than
under healthy conditions owing to hormonal stimulation. The rise in serum catecholamine levels increases plasma free fatty acid concentrations, and subsequently stimulates FAO68. As a
result, reducing the availability of circulating free fatty acids via transient adrenergic antagonists might be a viable therapy to inhibit FAO and increase glycolytic ATP production.
Traditionally, β-adrenergic receptor antagonists are used in HF owing to their negative ionotropic effects that reduce cardiac workload and spare oxygen by decreasing sympathetic activity68.
Many, such as carvedilol, have been clinically shown to lessen infarct size after ischaemia by decreasing sympathetic activity, followed by inhibition of mitochondrial fatty acid uptake and
increased glucose oxidation69. Malonyl-CoA endogenously regulates fatty acid concentrations by controlling the activity of carnitine _O_-palmitoyltransferase (CPT) 1, a rate-limiting enzyme
in mitochondrial fatty acid uptake68. When intracellular levels of malonyl-CoA are increased, CPT1 is inhibited and mitochondrial fatty acid uptake is stopped70. The intracellular
concentration of malonyl-CoA is dependent on the balance between its synthesis via acetyl-CoA carboxylase and degradation via malonyl-CoA decarboxylase. Therefore, the upregulation of
acetyl-CoA carboxylase or inhibition of malonyl-CoA decarboxylase would increase intracellular malonyl-CoA levels, and prevent mitochondrial uptake of free fatty acids to reduce FAO. As
expected, inhibiting malonyl-CoA decarboxylase in animal models has reportedly improved cardiac function after ischaemia, reduced cardiac FAO, and increased glycolytic flux71,72. Studies of
malonyl-CoA decarboxylase inhibitors in patients with HF are needed. Trends in glucose oxidation across the spectrum of HF are more variable, particularly among animal models of HF58.
Compensatory substrate switching towards glucose use has been observed in both animal models and humans59, with a higher contribution coming from glycolysis. Stimulating mitochondrial
glucose oxidation, either directly or by inhibiting fatty acid catabolism, has been suggested as a viable therapeutic strategy to compensate for the energetically 'starved' failing
heart59. Ketone body metabolism also seems to be altered in HF. Ketones are formed in the liver via fatty acid metabolism, and provide a small substrate pool for oxidation within the
myocardium. In conditions such as diabetes or starvation, ketone catabolism is upregulated in response to lowered insulin availability and higher fatty acid levels57,73. Studies have
reported increased ketone utilization in the severely failing heart in humans73,74. Further research is needed to understand the role of ketone oxidation in the failing myocardium, and to
determine whether targeting ketone metabolism is a plausible therapy to improve energetics in HF. Novel insights into the regulation of metabolic substrate demand in the heart have been
provided through studies of microRNAs and acetylation of mitochondrial lysine residues. Alterations in microRNA levels through any number of upregulation and downregulation events can alter
substrate utilization in the heart75. Alterations in protein levels modulated by microRNA expression have been proposed to have important implications for glycolysis, β-oxidation, ketone
metabolism, the Krebs cycle, and the electron transport chain (ETC)75. For example, increased levels of ROS can alter calcium handling in HF by modifying microRNA that leads to inhibition of
sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA) 2a transcription75. Post-translational modification via lysine acetylation has been suggested to have an important role in
metabolic enzyme regulation in the mitochondria59. OVERACTIVATION OF THE SNS As all substrates converge on mitochondria, understanding the specific abnormalities that occur in HF is central
to the development of new treatments. ROS production increases in many aetiologies of HF, a phenomenon that might be directly related to increased sympathetic nervous system (SNS) tone76.
Sustained sympathetic drive and chronically elevated circulating catecholamines — processes that are normally transient to mediate acute increases in cardiac output — are commonly observed
in patients with HF (particularly HFrEF)77,78. Chronic stimulation of β-adrenergic receptors has been directly linked to mitochondrial ROS production through adrenergic receptor-mediated
second messenger signalling79,80. ROS-mediated initiation of mitochondria-dependent cell death cascades has been repeatedly observed after chronic sympathetic activation, leading to overall
declines in mitochondrial function81,82,83,84,85,86. These processes can be amplified by the formation of aminochromes, catecholamine metabolites known to impair mitochondrial redox
balance87. Attenuation of HF pathology with β-blockers and renin–angiotensin–aldosterone antagonism has resulted in substantial clinical improvements88, and is likely to relieve some of the
mitochondrial dysfunction that accompanies increased sympathetic tone. The capacity to complement these existing background therapies with compounds that directly target mitochondrial
dysfunction is a potentially promising novel paradigm (Fig. 1). INCREASED ROS PRODUCTION Cellular ROS production occurs when ROS formation outpaces or exhausts compensatory signals and
overwhelms endogenous scavenging systems89,90,91. ROS are produced at several different sites within cells, both within and outside of mitochondria (reviewed in detail
previously92,93,94,95). Mitochondrial ROS production occurs at various sites along the inner mitochondrial membrane as well as in the mitochondrial matrix by components of the ETC and the
Krebs cycle, respectively96 (Fig. 4). ROS production is typically low under normal physiological conditions93, and is kept in check by intracellular and intramitochondrial scavenging
systems. Pathological ROS levels in the heart typically occur when ROS production outpaces endogenous scavenging capacity. ROS (and other associated reactive intermediates) can damage
proteins and lipids, trigger cell-death cascades, and evoke synchronized collapses in the cellular energy grid97,98. Heightened mitochondrial ROS production and downstream ROS-mediated
damage has been reported in patients with HF as well as in preclinical models of the disease31,99,100,101. Although ROS are typically associated with pathological states, ROS levels in the
heart _per se_ are best characterized by the term 'hormesis': small amounts can evoke adaptive signalling and create beneficial, compensatory responses. Modest production of ROS
has been shown to mediate beneficial myocardial signalling involved in physiological responses such as (transient) sympathetic drive102, many preconditioning paradigms103, cardiac
mitochondrial quality control104, and exercise105. Exercise training is known to augment endogenous ROS-scavenging mechanisms in the heart105,106,107, restore bioenergetic efficiency in
porcine models of HFpEF108, and improve symptoms and quality of life in trials involving patients with HFrEF109,110 or HFpEF111. Consistent with the ROS hormesis concept, several studies
have noted that administration of high doses of ROS scavengers can abolish the beneficial effects of exercise112,113, including humans taking oral vitamin C or E supplements114.
Mitochondrial production of ROS depends on the mitochondrial membrane potential. Increased expression of mitochondrial uncoupling proteins in HF115 might be a compensatory mechanism to
reduce ROS by 'uncoupling to survive'116, whereby a reduction in mitochondrial membrane potential is postulated to lower ROS emission from mitochondria. This view is popular and
almost dogmatic, but the decrease in ROS production by uncoupling is a prominent effect during mitochondrial state 4 respiration (no ADP). Heart mitochondria, however, are never respiring in
state 4. Pathological ROS production in cardiomyocytes is likely to be more closely linked to decreased or collapsed membrane potential and/or depletion of the NADPH pool117,118,119,
whereby ROS production overwhelms endogenous scavenging through mitochondrial membrane-dependent mechanisms89. The repeated lack of benefits of ROS scavenging compounds in clinical trials of
patients with HF11,120,121 continues to plague cardiovascular drug development, suggesting that oxygen radical scavenging _per se_ is not a plausible mechanism of action for long-term
improvements in HF. Lack of tissue permeability, poor intracellular targeting, and ineffective therapeutic doses might contribute to the poor translation of benefits of antioxidants to date.
This approach to therapy, however, might ultimately succeed when novel scavenging compounds that overcome permeability and targeting problems, such as XJB-5-131 (Refs 122,123),
mitoTEMPO124,125, and EUK8/EUK134 (Refs 126,127,128), are tested in humans. ABNORMALITIES OF MITOCHONDRIAL ETC Decrements in individual electron transport complexes, particularly complex I
and/or IV activity, have been observed in animal models129 and humans35 with HF. Electron transport system proteins seem to aggregate into functional supercomplexes130,131,132, and a loss of
mitochondrial supercomplexes, which is postulated to have a causal role in mitochondrial ROS generation133, has been noted in HF134. Several approaches are being developed to improve the
efficiency of the ETC in HF. The coenzyme Q (ubiquinol/ubiquinone CoQ) pool comprises a redox-cycling coenzyme found in the ETC. CoQ is typically synthesized _de novo_ and undergoes a
two-electron reduction from substrates fed into complexes I and II, and is then oxidized as it donates electrons into complex III. As a redox cycler, the ubiquinol/ubiquinone couple can both
accept and donate electrons, depending on the redox potential135. Incomplete, one-electron reduction of CoQ produces semiquinone, itself a highly reactive radical. A reduced CoQ pool could
potentially feed electrons 'backwards' towards complex I, which results in reverse electron transfer and ROS generation136. Decreased circulating CoQ has been observed in patients
with HF137,138, with an inverse correlation observed between plasma CoQ and mortality139. In the Q-SYMBIO trial140, the efficacy of CoQ was tested in a small (_n_ = 420), double-blind,
placebo-controlled study in patients with HF and showed a reduction in mortality after 2 years of treatment. Although the Q-SYMBIO trial was fairly small, the promising findings triggered
interest in the development of other CoQ analogues that more effectively target mitochondria. New quinone conjugates that are tethered to lipophilic, cationic triphenylphosphonium moieties,
such as MitoQ, SkQ, and other plastoquinones, might improve the delivery of CoQ to mitochondria141,142,143, and have shown some promise in preclinical models of HF144. A potential problem
with the use of these compounds is that they are self-limiting, in that they can depolarize mitochondria and inhibit mitochondrial respiration at high concentrations145. Several short-chain
synthetic CoQ analogues are also in development, including EPI-743 (Ref. 146) and idebenone147. These compounds have shown promise in small trials of genetic mitochondrial disease148,149,
but have not yet been tested in larger trials of human HF. Aberrant mitochondrial membrane phospholipids in HF are integrally involved in ETC dysfunction. A membrane phospholipid integral to
optimal function of the ETC and whose content and composition are altered in HF is cardiolipin. Cardiolipin resides in the inner mitochondrial membrane (Fig. 4) and, unlike most
phospholipids that have two acyl tails, cardiolipin has four acyl chains. In mammalian hearts, these chains are enriched with linoleic acid (18:2)4. Cardiolipin decrements are observed in
both paediatric150 and adult151,152 patients with HF. Cardiolipin is essential for the activity of ETC complexes, membrane transporters, mitochondrial ion homeostasis, and ROS production153.
Given that most mitochondrial complexes associated with energy production are oligomers composed of many subunits, cardiolipin is proposed to act as molecular 'glue' holding these
subunits together154,155,156. Approaches that target cardiolipin are likely to improve electron transport across the ETC and, in doing so, might be beneficial in treating HF. A compound
that targets cardiolipin in the mitochondria that is currently in clinical development is the cell-permeable peptide MTP-131 (also called elamipretide or Bendavia). An analogue of MTP-131
(SS-31) was serendipitously discovered by Szeto and Schiller in attempts to identify small peptides with opioid-receptor binding properties157. MTP-131 has no discernible opioid-receptor
activity158, but was found to localize to the inner mitochondrial membrane159, reduce myocardial ischaemia–reperfusion injury112,160,161, improve renal function51,162, and restore skeletal
muscle function163. MTP-131 is not a direct ROS scavenger164, and is postulated to act by interacting with cardiolipin165 to interrupt the vicious cycle of ROS-mediated cardiolipin oxidation
and subsequent loss of energetics119,166. MTP-131-mediated improvements in mitochondrial energetics have been observed across a number of different tissues in animal models of disease,
including the myocardium161,163,164. Of note, MTP-131 can improve mitochondrial bioenergetics by improving respiratory supercomplex formation (D. A. Brown, unpublished work). MTP-131 is
currently being investigated in several phase II clinical trials. Preclinical studies in mouse models of HF have demonstrated efficacy using MTP-131. In a mouse model of HF induced by aortic
constriction, MTP-131 improved left ventricular function, reduced hypertrophic remodelling, and restored mitochondrial function167. In complementary studies, MTP-131 administration
substantially reduced maladaptive remodelling, preserved cardiac function, lowered β-adrenergic-mediated calcium overload, and restored mitochondrial protein expression168,169,170. A
substantial improvement in cardiac function with MTP-131 has been demonstrated in a porcine model of HFpEF171 and a canine model of HFrEF172. Beneficial improvements in ejection fraction
were associated with improved activity or expression of mitochondrial complexes I, IV, and V, and a normalization of cardiolipin levels172. As the HF syndrome influences many different
tissues (Fig. 3), the evidence that MTP-131 also improves skeletal muscle function, exercise capacity, and renal function adds to the promise of this emerging therapy51,163,173,174. BLOCKERS
OF THE MPTP The mitochondrial permeability transition pore (MPTP) is a nonspecific pore that opens in response to increased calcium levels and oxidative challenge, and is associated with
ROS production, apoptotic cell death, and mitochondrial dysfunction. Increased proclivity of MPTP opening occurs in both acute and chronic heart disease, and numerous preclinical studies
have demonstrated efficacy in cardiac pathology with MPTP blockers, such as cyclosporin, NIM811, and TRO40303 (reviewed previously175,176,177,178,179). Although the opening of the MPTP has
historically been thought of as a pathological event leading to cell death, studies now suggest that transient MPTP opening might be a physiological 'reset' mechanism to prevent
mitochondrial calcium overload. Rare, transient openings of the MPTP have been observed in individual mitochondria of primary cardiomyocytes180. Small, brief MPTP openings were found to be
more frequent in HF cardiomyocytes, and were associated with transient mitochondrial depolarization and mitochondrial calcium release. If opening of these pores might be a normal
compensatory mechanism akin to 'pressure release valves', the concept of treating HF by blocking them becomes increasingly difficult. Ongoing uncertainty regarding the molecular
identity of the MPTP further complicates the development of novel therapies that act on the pore176,181,182,183,184,185. The MPTP seems to be comprised of ATP synthase (complex V) dimers and
to be gated by mitochondrial matrix calcium content via cyclophillin D186,187. Clinical studies have failed to demonstrate efficacy in most188,189, but not all190,191, studies; however,
most of these studies focused on reducing acute cardiac ischaemia–reperfusion injury and not in limiting left ventricular dysfunction in HF. Chronic administration of cyclosporin has been
linked with renal pathology and immunosuppression192,193, and cyclosporin was found to evoke systemic hypertension in porcine models of HFpEF194. Accordingly, cyclosporin is not an
appropriate approach for the long-term management of HF. Further work with alternative MPTP blockers is needed to determine whether inhibiting or delaying MPTP opening is a clinically
plausible approach to alter the progression of HF. CELLULAR/MITOCHONDRIAL ION HOMEOSTASIS Aberrant handling of several different ions within the mitochondria has been observed, mostly in
animal models of HF. Heightened levels of free iron can increase ROS through Fenton chemistry. Changes in cellular iron handling have been noted in HF7,195, and orally-available iron
chelators such as deferiprone seem to redistribute iron from tissues, including the mitochondrial space, into the circulation196. Although a potential exists to treat HF by chelating
cellular iron, no study to date has shown functional improvements of the failing heart, although several clinical trials are currently underway. Impaired cellular calcium handling that leads
to decrements in excitation–contraction coupling is noted across HF aetiologies, and contributes to poor cardiac mechanics and to arrhythmogenesis197,198,199,200. Mitochondria can directly
influence cellular calcium dynamics, because many of the membrane-bound pumps required for cytosolic calcium release and removal are energy-dependent and ROS-dependent. Altered calcium
handling has been implicated in HFpEF, in which abnormal calcium dynamics impair relaxation. Short-term administration of ivabradine to slow the heart rate led to modest benefits in patients
with HFpEF, ostensibly by providing more time for calcium-dependent relaxation201. The vast majority of calcium resequestration into the sarcoplasmic reticulum, obligatory for diastolic
relaxation, occurs through SERCA2a, which has been shown to be downregulated in HF202,203,204. Overexpressing SERCA2a has shown promise in animal models of HF205,206, although several
barriers (such as the development of neutralizing antibodies) still exist before gene transfer realizes its full translational potential207. Furthermore, increased ROS can oxidize proteins
associated with the ryanodine receptor calcium-release channel, which can lead to calcium leaking out of the sarcoplasmic reticulum during diastole208. Increased intracellular sodium levels
in HF209,210,211,212 also contribute to poor calcium handling through mechanisms involving sodium–calcium exchange. Given that calcium is central to maintaining bioenergetic supply–demand
matching21,213, sodium overload alters cellular and mitochondrial calcium fluxes and impairs bioenergetic supply–demand matching in HF214. Although very early in development, inhibitors of
the mitochondrial sodium–calcium–(lithium) exchanger215, such as CGP-37157, have been shown to improve cardiac function in preclinical models of HF216,217. Inhibiting the sarcolemmal
sodium–calcium exchanger might also be a promising approach, as demonstrated in a preclinical model of HFpEF218. Another compound in clinical development to improve cardiac efficiency in HF
is omecamtiv mecarbil (CK-1827452). This drug increases the calcium sensitivity of the myofilaments219, which prolongs the duration of systole in animal models and in human HF220,221,222.
Two substantial phase IIb, double-blind, randomized studies comparing omecamtiv mecarbil and placebo have been conducted. In the ATOMIC-HF trial223, omecamtiv mecarbil was administered for
48 h intravenously to patients with acute HF. Overall, the study was neutral (with some evidence of a symptomatic benefit at higher doses), but suggested omecamtiv mecarbil was safe. In the
COSMIC-HF trial224, an oral formulation of omecamtiv mecarbil was associated with improvements in cardiac function over 20 weeks, with an effect that persisted for 4 weeks after stopping the
drug, suggesting that improved function had produced favourable structural remodelling. Despite the promise of omecamtiv mecarbil, concerns about elevated levels of serum troponin225,
metabolic inefficiency226, and impaired cardiac relaxation227 must be assuaged by larger clinical trials to understand fully whether this approach can improve prognosis in HF. CONCLUSIONS
The vast majority of HF trials over the past decade have been neutral, and event rates remain unacceptably high. Perhaps most alarming, no proven therapies exist for patients with worsening
chronic HF or HFpEF — populations that collectively comprise the majority of the total HF population. Moreover, although systemic blockade of maladaptive neurohormonal responses has improved
outcomes in HFrEF, these agents also lower blood pressure and/or heart rate, and development of new haemodynamically active drugs for stepwise addition to existing therapies raises safety
and tolerability concerns. Therefore, an ideal novel therapy would be haemodynamically neutral and target the myocardium as the centrepiece of the therapeutic mechanism. In this context,
overwhelming evidence from both preclinical and clinical studies indicates bioenergetic insufficiency in HF. Studies using preclinical models of the disease continue to advance our
understanding of the cellular and molecular mechanisms that contribute to poor bioenergetics of the failing heart. Considerable potential exists to fill this unmet need, mitigate the
economic burdens, and reduce symptoms in patients with HF by focusing on the development of new therapeutic modalities that target mitochondrial abnormalities in HF. REFERENCES * Jessup, M.
_ et al_. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart
Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. _Circulation_ 119, 1977–2016 (2009). Article
PubMed Google Scholar * Hunt, S. A. _ et al_. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of
Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in
collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. _Circulation_ 112,
e154–e235 (2005). Article PubMed Google Scholar * Wilcox, J. E. _ et al_. “Targeting the Heart” in heart failure: myocardial recovery in heart failure with reduced ejection fraction.
_JACC Heart Fail._ 3, 661–669 (2015). Article PubMed Google Scholar * Braunschweig, F., Cowie, M. R. & Auricchio, A. What are the costs of heart failure? _Europace_ 13, ii13–ii17
(2011). Article PubMed Google Scholar * Yancy, C. W. _ et al_. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology
Foundation/American Heart Association Task Force on Practice Guidelines. _J. Am. Coll. Cardiol._ 62, e147–e239 (2013). Article PubMed Google Scholar * Meta-analysis Global Group in
Chronic Heart Failure. The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. _Eur. Heart J._ 33,
1750–1757 (2012). * Bayeva, M., Gheorghiade, M. & Ardehali, H. Mitochondria as a therapeutic target in heart failure. _J. Am. Coll. Cardiol._ 61, 599–610 (2013). Article CAS PubMed
Google Scholar * Neely, J. R., Liebermeister, H., Battersby, E. J. & Morgan, H. E. Effect of pressure development on oxygen consumption by isolated rat heart. _Am. J. Physiol._ 212,
804–814 (1967). Article CAS PubMed Google Scholar * Gheorghiade, M. _ et al_. Developing new treatments for heart failure: focus on the heart. _Circ. Heart Fail._ 9, e002727 (2016).
Article PubMed Google Scholar * Vaduganathan, M., Butler, J., Pitt, B. & Gheorghiade, M. Contemporary drug development in heart failure: call for hemodynamically neutral therapies.
_Circ. Heart Fail._ 8, 826–831 (2015). Article PubMed Google Scholar * Downey, J. M. & Cohen, M. V. Why do we still not have cardioprotective drugs? _Circ. J._ 73, 1171–1177 (2009).
Article PubMed Google Scholar * Senni, M., Gavazzi, A., Gheorghiade, M. & Butler, J. Heart failure at the crossroads: moving beyond blaming stakeholders to targeting the heart. _Eur.
J. Heart Fail._ 17, 760–763 (2015). Article PubMed Google Scholar * Fordyce, C. B. _ et al_. Cardiovascular drug development: is it dead or just hibernating? _J. Am. Coll. Cardiol._ 65,
1567–1582 (2015). Article PubMed Google Scholar * Amidon, S. & Amidon, T. _The Sublime Engine: A Biography of the Human Heart_. (Rodale Books, 2011). Google Scholar *
Tornroth-Horsefield, S. & Neutze, R. Opening and closing the metabolite gate. _Proc. Natl Acad. Sci. USA_ 105, 19565–19566 (2008). Article PubMed CAS PubMed Central Google Scholar *
Balaban, R. S. Cardiac energy metabolism homeostasis: role of cytosolic calcium. _J. Mol. Cell. Cardiol._ 34, 1259–1271 (2002). Article CAS PubMed Google Scholar * Barth, E., Stammler,
G., Speiser, B. & Schaper, J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. _J. Mol. Cell. Cardiol._ 24,
669–681 (1992). Article CAS PubMed Google Scholar * Schaper, J., Meiser, E. & Stammler, G. Ultrastructural morphometric analysis of myocardium from dogs, rats, hamsters, mice, and
from human hearts. _Circ. Res._ 56, 377–391 (1985). Article CAS PubMed Google Scholar * Opie, L. _The Heart: Physiology, from Cell to Circulation_ 3rd edn, (Lipincott−Raven, 1998).
Google Scholar * Balaban, R. S. Domestication of the cardiac mitochondrion for energy conversion. _J. Mol. Cell. Cardiol._ 6, 832–841 (2009). Article CAS Google Scholar * Balaban, R. S.
The role of Ca2+ signaling in the coordination of mitochondrial ATP production with cardiac work. _Biochim. Biophys. Acta_ 1787, 1334–1341 (2009). Article CAS PubMed PubMed Central
Google Scholar * Suliman, H. B. & Piantadosi, C. A. Mitochondrial quality control as a therapeutic target. _Pharmacol. Rev._ 68, 20–48 (2016). Article CAS PubMed Google Scholar *
Lesnefsky, E. J., Chen, Q. & Hoppel, C. L. Mitochondrial metabolism in aging heart. _Circ. Res._ 118, 1593–1611 (2016). Article CAS PubMed PubMed Central Google Scholar * Gottlieb,
R. A. & Bernstein, D. Mitochondrial remodeling: rearranging, recycling, and reprogramming. _Cell Calcium_ 60, 88–101 (2016). Article CAS PubMed PubMed Central Google Scholar *
Shirihai, O. S., Song, M. & Dorn, G. W. II. How mitochondrial dynamism orchestrates mitophagy. _Circ. Res._ 116, 1835–1849 (2015). Article CAS PubMed PubMed Central Google Scholar *
Dorn, G. W., II & Kitsis, R. N. The mitochondrial dynamism-mitophagy-cell death interactome: multiple roles performed by members of a mitochondrial molecular ensemble. _Circ. Res._ 116,
167–182 (2015). Article CAS PubMed Google Scholar * Biala, A. K., Dhingra, R. & Kirshenbaum, L. A. Mitochondrial dynamics: orchestrating the journey to advanced age. _J. Mol. Cell.
Cardiol._ 83, 37–43 (2015). Article CAS PubMed Google Scholar * Dhingra, R. & Kirshenbaum, L. A. Regulation of mitochondrial dynamics and cell fate. _Circ. J._ 78, 803–810 (2014).
Article CAS PubMed Google Scholar * Thomas, R. L. & Gustafsson, A. B. Mitochondrial autophagy — an essential quality control mechanism for myocardial homeostasis. _Circ. J._ 77,
2449–2454 (2013). CAS PubMed PubMed Central Google Scholar * Sebastiani, M. _ et al_. Induction of mitochondrial biogenesis is a maladaptive mechanism in mitochondrial cardiomyopathies.
_J. Am. Coll. Cardiol._ 50, 1362–1369 (2007). Article CAS PubMed Google Scholar * Goh, K. Y. _ et al_. Impaired mitochondrial network excitability in failing guinea-pig cardiomyocytes.
_Cardiovasc. Res._ 109, 79–89 (2016). Article CAS PubMed Google Scholar * Sabbah, H. N. _ et al_. Mitochondrial abnormalities in myocardium of dogs with chronic heart failure. _J. Mol.
Cell. Cardiol._ 24, 1333–1347 (1992). Article CAS PubMed Google Scholar * Lehman, J. J. & Kelly, D. P. Gene regulatory mechanisms governing energy metabolism during cardiac
hypertrophic growth. _Heart Fail. Rev._ 7, 175–185 (2002). Article CAS PubMed Google Scholar * Lai, L. _ et al_. Energy metabolic reprogramming in the hypertrophied and early stage
failing heart: a multisystems approach. _Circ. Heart Fail._ 7, 1022–1031 (2014). Article CAS PubMed PubMed Central Google Scholar * Lemieux, H., Semsroth, S., Antretter, H., Hofer, D.
& Gnaiger, E. Mitochondrial respiratory control and early defects of oxidative phosphorylation in the failing human heart. _Int. J. Biochem. Cell Biol._ 43, 1729–1738 (2011). Article
CAS PubMed Google Scholar * Nicholls, D. G. & Ferguson, S. J. _Bioenergetics_ 3rd edn (Academic, 2002). Google Scholar * Schon, E. A., DiMauro, S. & Hirano, M. Human
mitochondrial DNA: roles of inherited and somatic mutations. _Nat. Rev. Genet._ 13, 878–890 (2012). Article CAS PubMed PubMed Central Google Scholar * Ott, M., Amunts, A. & Brown,
A. Organization and regulation of mitochondrial protein synthesis. _Annu. Rev. Biochem._ 85, 77–101 (2016). Article CAS PubMed Google Scholar * Bates, M. G. _ et al_. Cardiac involvement
in mitochondrial DNA disease: clinical spectrum, diagnosis, and management. _Eur. Heart J._ 33, 3023–3033 (2012). Article CAS PubMed PubMed Central Google Scholar * Margulis, L.
Symbiotic theory of the origin of eukaryotic organelles; criteria for proof. _Symp. Soc. Exp. Biol._ 29 21–38 (1975). Google Scholar * Sato, M. & Sato, K. Maternal inheritance of
mitochondrial DNA by diverse mechanisms to eliminate paternal mitochondrial DNA. _Biochim. Biophys. Acta_ 1833, 1979–1984 (2013). Article CAS PubMed Google Scholar * Taylor, R. W. &
Turnbull, D. M. Mitochondrial DNA mutations in human disease. _Nat. Rev. Genet._ 6, 389–402 (2005). Article CAS PubMed PubMed Central Google Scholar * Bacman, S. R., Williams, S. L.,
Pinto, M., Peralta, S. & Moraes, C. T. Specific elimination of mutant mitochondrial genomes in patient-derived cells by mitoTALENs. _Nat. Med._ 19, 1111–1113 (2013). Article CAS PubMed
PubMed Central Google Scholar * Reddy, P. _ et al_. Selective elimination of mitochondrial mutations in the germline by genome editing. _Cell_ 161, 459–469 (2015). Article CAS PubMed
PubMed Central Google Scholar * Paull, D. _ et al_. Nuclear genome transfer in human oocytes eliminates mitochondrial DNA variants. _Nature_ 493, 632–637 (2013). Article CAS PubMed
Google Scholar * Mitchell, P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. _Nature_ 191, 144–148 (1961). Article CAS PubMed Google
Scholar * Carrasco, A. J. _ et al_. Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. _Proc. Natl Acad. Sci. USA_ 98, 7623–7628
(2001). Article CAS PubMed PubMed Central Google Scholar * Wu, F., Zhang, J. & Beard, D. A. Experimentally observed phenomena on cardiac energetics in heart failure emerge from
simulations of cardiac metabolism. _Proc. Natl Acad. Sci. USA_ 106, 7143–7148 (2009). Article CAS PubMed PubMed Central Google Scholar * Neubauer, S. _ et al_. Downregulation of the
Na+-creatine cotransporter in failing human myocardium and in experimental heart failure. _Circulation_ 100, 1847–1850 (1999). Article CAS PubMed Google Scholar * Abozguia, K. _ et al_.
Reduced _in vivo_ skeletal muscle oxygen consumption in patients with chronic heart failure — a study using Near Infrared Spectrophotometry (NIRS). _Eur. J. Heart Fail._ 10, 652–657 (2008).
Article CAS PubMed Google Scholar * Eirin, A. _ et al_. Mitochondrial protection restores renal function in swine atherosclerotic renovascular disease. _Cardiovasc. Res._ 103, 461–472
(2014). Article CAS PubMed PubMed Central Google Scholar * Anderson, E. J. _ et al_. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in
both rodents and humans. _J. Clin. Invest._ 3, 573–581 (2009). Article CAS Google Scholar * Zhang, Q. _ et al_. Catechin ameliorates cardiac dysfunction in rats with chronic heart
failure by regulating the balance between Th17 and Treg cells. _Inflamm. Res._ 63, 619–628 (2014). Article CAS PubMed Google Scholar * Ramirez-Sanchez, I. _ et al_. (−)-Epicatechin rich
cocoa mediated modulation of oxidative stress regulators in skeletal muscle of heart failure and type 2 diabetes patients. _Int. J. Cardiol._ 168, 3982–3990 (2013). Article PubMed PubMed
Central Google Scholar * Sung, M. M. & Dyck, J. R. Therapeutic potential of resveratrol in heart failure. _Ann. NY Acad. Sci._ 1348, 32–45 (2015). Article CAS PubMed Google Scholar
* Magyar, K. _ et al_. Cardioprotection by resveratrol: a human clinical trial in patients with stable coronary artery disease. _Clin. Hemorheol. Microcirc._ 50, 179–187 (2012). CAS
PubMed Google Scholar * Stanley, W. C., Recchia, F. A. & Lopaschuk, G. D. Myocardial substrate metabolism in the normal and failing heart. _Physiol. Rev._ 85, 1093–1129 (2005). Article
CAS PubMed Google Scholar * Doenst, T., Nguyen, T. D. & Abel, E. D. Cardiac metabolism in heart failure: implications beyond ATP production. _Circ. Res._ 113, 709–724 (2013).
Article CAS PubMed PubMed Central Google Scholar * Fukushima, A., Milner, K., Gupta, A. & Lopaschuk, G. D. Myocardial energy substrate metabolism in heart failure: from pathways to
therapeutic targets. _Curr. Pharm. Des._ 21, 3654–3664 (2015). Article CAS PubMed Google Scholar * Ventura-Clapier, R., Garnier, A. & Veksler, V. Energy metabolism in heart failure.
_J. Physiol._ 555, 1–13 (2004). Article CAS PubMed Google Scholar * Aubert, G., Vega, R. B. & Kelly, D. P. Perturbations in the gene regulatory pathways controlling mitochondrial
energy production in the failing heart. _Biochim. Biophys. Acta_ 1833, 840–847 (2013). Article CAS PubMed Google Scholar * de las Fuentes, L. _ et al_. Myocardial fatty acid metabolism:
independent predictor of left ventricular mass in hypertensive heart disease. _Hypertension_ 41, 83–87 (2003). Article CAS PubMed Google Scholar * Goikoetxea, M. J. _ et al_. Altered
cardiac expression of peroxisome proliferator-activated receptor-isoforms in patients with hypertensive heart disease. _Cardiovasc. Res._ 69, 899–907 (2006). Article CAS PubMed Google
Scholar * Sack, M. N. _ et al_. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. _Circulation_ 94, 2837–2842 (1996). Article CAS PubMed Google Scholar
* Sihag, S., Cresci, S., Li, A. Y., Sucharov, C. C. & Lehman, J. J. _PGC-1 α_ and _ERR α_ target gene downregulation is a signature of the failing human heart. _J. Mol. Cell. Cardiol._
46, 201–212 (2009). Article CAS PubMed Google Scholar * Lehman, J. J. _ et al_. Peroxisome proliferator-activated receptor γ coactivator-1 promotes cardiac mitochondrial biogenesis. _J.
Clin. Invest._ 106, 847–856 (2000). CAS PubMed PubMed Central Google Scholar * Sarma, S., Ardehali, H. & Gheorghiade, M. Enhancing the metabolic substrate: PPAR-alpha agonists in
heart failure. _Heart Fail. Rev._ 17, 35–43 (2012). Article CAS PubMed Google Scholar * Jaswal, J. S., Keung, W., Wang, W., Ussher, J. R. & Lopaschuk, G. D. Targeting fatty acid and
carbohydrate oxidation — a novel therapeutic intervention in the ischemic and failing heart. _Biochim. Biophys. Acta_ 1813, 1333–1350 (2011). Article CAS PubMed Google Scholar *
Igarashi, N. _ et al_. Influence of β-adrenoceptor blockade on the myocardial accumulation of fatty acid tracer and its intracellular metabolism in the heart after ischemia−reperfusion
injury. _Circ. J._ 70, 1509–1514 (2006). Article CAS PubMed Google Scholar * Fillmore, N. & Lopaschuk, G. D. Malonyl CoA: a promising target for the treatment of cardiac disease.
_IUBMB Life_ http://dx.doi.org/10.1002/iub.1253 (2014). * Stanley, W. C. _ et al_. Malonyl-CoA decarboxylase inhibition suppresses fatty acid oxidation and reduces lactate production during
demand-induced ischemia. _Am. J. Physiol. Heart Circ. Physiol._ 289, H2304–H2309 (2005). Article CAS PubMed Google Scholar * Dyck, J. R. _ et al_. Malonyl coenzyme A decarboxylase
inhibition protects the ischemic heart by inhibiting fatty acid oxidation and stimulating glucose oxidation. _Circ. Res._ 94, e78–e84 (2004). Article CAS PubMed Google Scholar * Kolwicz,
S. C. Jr., Airhart, S. & Tian, R. Ketones step to the plate: a game changer for metabolic remodeling in heart failure? _Circulation_ 133, 689–691 (2016). Article PubMed PubMed Central
Google Scholar * Bedi, K. C. Jr. _ et al_. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure.
_Circulation_ 133, 706–716 (2016). Article CAS PubMed PubMed Central Google Scholar * Pinti, M. V., Hathaway, Q. A. & Hollander, J. M. Role of microRNA in metabolic shift during
heart failure. _Am. J. Physiol. Heart Circ. Physiol._ http://dx.doi.org/10.1152/ajpheart.00341.2016 (2016). * Opie, L. H., Thandroyen, F. T., Muller, C. & Bricknell, O. L.
Adrenaline-induced “oxygen-wastage” and enzyme release from working rat heart. Effects of calcium antagonism, β-blockade, nicotinic acid and coronary artery ligation. _J. Mol. Cell.
Cardiol._ 11, 1073–1094 (1979). Article CAS PubMed Google Scholar * Francis, G. S. _ et al_. Plasma norepinephrine, plasma renin activity, and congestive heart failure. Relations to
survival and the effects of therapy in V-HeFT II. The V-HeFT VA Cooperative Studies Group. _Circulation_ 87, VI40–V148 (1993). CAS PubMed Google Scholar * Triposkiadis, F. _ et al_. The
sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. _J. Am. Coll. Cardiol._ 54, 1747–1762 (2009). Article CAS PubMed Google Scholar *
Menon, B. _ et al_. Expression of the cytoplasmic domain of β1 integrin induces apoptosis in adult rat ventricular myocytes (ARVM) via the involvement of caspase-8 and mitochondrial death
pathway. _Basic Res. Cardiol._ 101, 485–493 (2006). Article CAS PubMed Google Scholar * Rosca, M. G. & Hoppel, C. L. Mitochondrial dysfunction in heart failure. _Heart Fail. Rev._
18, 607–622 (2013). Article CAS PubMed Google Scholar * Leger, B. _ et al_. Chronic formoterol administration reduces cardiac mitochondrial protein synthesis and oxidative capacity in
mice. _Int. J. Cardiol._ 146, 270–272 (2011). Article PubMed Google Scholar * Izem-Meziane, M. _ et al_. Catecholamine-induced cardiac mitochondrial dysfunction and mPTP opening:
protective effect of curcumin. _Am. J. Physiol. Heart Circ. Physiol._ 302, H665–674 (2012). Article CAS PubMed Google Scholar * Nagasaka, S. _ et al_. Protein kinase A catalytic subunit
alters cardiac mitochondrial redox state and membrane potential via the formation of reactive oxygen species. _Circ. J._ 71, 429–436 (2007). Article CAS PubMed Google Scholar *
Remondino, A. _ et al_. β-adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the
mitochondrial pathway. _Circ. Res._ 92, 136–138 (2003). Article CAS PubMed Google Scholar * Communal, C., Colucci, W. S. & Singh, K. p38 mitogen-activated protein kinase pathway
protects adult rat ventricular myocytes against β -adrenergic receptor-stimulated apoptosis. Evidence for Gi-dependent activation. _J. Biol. Chem._ 275, 19395–19400 (2000). Article CAS
PubMed Google Scholar * Communal, C., Singh, K., Sawyer, D. B. & Colucci, W. S. Opposing effects of β1- and β2-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis
toxin-sensitive G protein. _Circulation_ 100, 2210–2212 (1999). Article CAS PubMed Google Scholar * Liaudet, L., Calderari, B. & Pacher, P. Pathophysiological mechanisms of
catecholamine and cocaine-mediated cardiotoxicity. _Heart Fail. Rev._ 19, 815–824 (2014). Article CAS PubMed Google Scholar * Feldman, D. S., Carnes, C. A., Abraham, W. T. & Bristow,
M. R. Mechanisms of disease: β-adrenergic receptors — alterations in signal transduction and pharmacogenomics in heart failure. _Nat. Clin. Pract. Cardiovasc. Med._ 2, 475–483 (2005).
Article CAS PubMed Google Scholar * Aon, M. A., Cortassa, S. & O'Rourke, B. Redox-optimized ROS balance: a unifying hypothesis. _Biochim. Biophys. Acta_ 6, 865–877 (2010).
Article CAS Google Scholar * Brown, D. A., Sabbah, H. N. & Shaikh, S. R. Mitochondrial inner membrane lipids and proteins as targets for decreasing cardiac ischemia/reperfusion
injury. _Pharmacol. Ther._ 140, 258–266 (2013). Article CAS PubMed Google Scholar * Murphy, E. & Steenbergen, C. Mechanisms underlying acute protection from cardiac
ischemia−reperfusion injury. _Physiol. Rev._ 88, 581–609 (2008). Article CAS PubMed Google Scholar * Murphy, E. & Steenbergen, C. Ion transport and energetics during cell death and
protection. _Physiology (Bethesda)_ 23, 115–123 (2008). CAS Google Scholar * Murphy, M. P. How mitochondria produce reactive oxygen species. _Biochem. J._ 417, 1–13 (2009). Article CAS
PubMed Google Scholar * Walters, A. M., Porter, G. A. Jr & Brookes, P. S. Mitochondria as a drug target in ischemic heart disease and cardiomyopathy. _Circ. Res._ 111, 1222–1236
(2012). Article CAS PubMed PubMed Central Google Scholar * Nabeebaccus, A., Zhang, M. & Shah, A. M. NADPH oxidases and cardiac remodelling. _Heart Fail. Rev._ 16, 5–12 (2011).
Article CAS PubMed Google Scholar * Orr, A. L. _ et al_. Inhibitors of ROS production by the ubiquinone-binding site of mitochondrial complex I identified by chemical screening. _Free
Radic. Biol. Med._ 65, 1047–1059 (2013). Article CAS PubMed PubMed Central Google Scholar * Zorov, D. B., Juhaszova, M. & Sollott, S. J. Mitochondrial ROS-induced ROS release: an
update and review. _Biochim. Biophys. Acta_ 1757, 509–517 (2006). Article CAS PubMed Google Scholar * Aon, M. A., Cortassa, S., Akar, F. G. & O'Rourke, B. Mitochondrial
criticality: a new concept at the turning point of life or death. _Biochim. Biophys. Acta_ 1762, 232–240 (2006). Article CAS PubMed Google Scholar * Ide, T. _ et al_. Mitochondrial DNA
damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. _Circ. Res._ 88, 529–535 (2001). Article CAS PubMed Google Scholar * Ide, T. _ et
al_. Direct evidence for increased hydroxyl radicals originating from superoxide in the failing myocardium. _Circ. Res._ 86, 152–157 (2000). Article CAS PubMed Google Scholar * Ide, T. _
et al_. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. _Circ. Res._ 85, 357–363 (1999). Article CAS PubMed Google
Scholar * Rosca, M. G., Tandler, B. & Hoppel, C. L. Mitochondria in cardiac hypertrophy and heart failure. _J. Mol. Cell. Cardiol._ 55, 31–41 (2013). Article CAS PubMed Google
Scholar * Alleman, R. J., Katunga, L. A., Nelson, M. A., Brown, D. A. & Anderson, E. J. The “Goldilocks Zone” from a redox perspective — adaptive versus deleterious responses to
oxidative stress in striated muscle. _Front. Physiol._ 5, 358 (2014). Article PubMed PubMed Central Google Scholar * Song, M. _ et al_. Super-suppression of mitochondrial reactive oxygen
species signaling impairs compensatory autophagy in primary mitophagic cardiomyopathy. _Circ. Res._ 115, 348–353 (2014). Article CAS PubMed PubMed Central Google Scholar * Frasier, C.
R., Moore, R. L. & Brown, D. A. Exercise-induced cardiac preconditioning: how exercise protects your achy-breaky heart. _J. Appl. Physiol._ 111, 905–915 (2011). Article CAS PubMed
Google Scholar * Brown, D. A., Jew, K. N., Sparagna, G. C., Musch, T. I. & Moore, R. L. Exercise training preserves coronary flow and reduces infarct size following ischemia−reperfusion
in rat heart. _J. Appl. Physiol._ 95, 2510–2518 (2003). Article PubMed Google Scholar * Brown, D. A. & Moore, R. L. Perspectives in innate and acquired cardioprotection:
cardioprotection acquired through exercise. _J. Appl. Physiol._ 103, 1894–1899 (2007). Article CAS PubMed Google Scholar * Marshall, K. D. _ et al_. Heart failure with preserved ejection
fraction: chronic low-intensity interval exercise training preserves myocardial O2 balance and diastolic function. _J. Appl. Physiol._ 114, 131–147 (2013). Article PubMed Google Scholar
* Flynn, K. E. _ et al_. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. _JAMA_ 301, 1451–1459 (2009). Article
CAS PubMed PubMed Central Google Scholar * O'Connor, C. M. _ et al_. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled
trial. _JAMA_ 301, 1439–1450 (2009). Article CAS PubMed PubMed Central Google Scholar * Edelmann, F. _ et al_. Exercise training improves exercise capacity and diastolic function in
patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. _J. Am. Coll. Cardiol._ 58, 1780–1791 (2011).
Article PubMed Google Scholar * Frasier, C. R. _ et al_. Redox-dependent increases in glutathione reductase and exercise preconditioning: role of NADPH oxidase and mitochondria.
_Cardiovasc. Res._ 98, 47–55 (2013). Article CAS PubMed Google Scholar * Nelson, M. J., Harris, M. B., Boluyt, M. O., Hwang, H. S. & Starnes, J. W. Effect of N-2-mercaptopropionyl
glycine on exercise-induced cardiac adaptations. _Am. J. Physiol. Regul. Integr. Comp. Physiol._ 300, R993–R1000 (2011). Article CAS PubMed Google Scholar * Ristow, M. _ et al_.
Antioxidants prevent health-promoting effects of physical exercise in humans. _Proc. Natl Acad. Sci. USA_ 106, 8665–8670 (2009). Article CAS PubMed PubMed Central Google Scholar *
Akhmedov, A. T., Rybin, V. & Marin-Garcia, J. Mitochondrial oxidative metabolism and uncoupling proteins in the failing heart. _Heart Fail. Rev._ 20, 227–249 (2015). Article CAS PubMed
Google Scholar * Brand, M. D. Uncoupling to survive? The role of mitochondrial inefficiency in ageing. _Exp. Gerontol._ 35, 811–820 (2000). Article CAS PubMed Google Scholar *
Slodzinski, M. K., Aon, M. A. & O'Rourke, B. Glutathione oxidation as a trigger of mitochondrial depolarization and oscillation in intact hearts. _J. Mol. Cell. Cardiol._ 45,
650–660 (2008). Article CAS PubMed PubMed Central Google Scholar * Brown, D. A. _ et al_. Cardiac arrhythmias induced by glutathione oxidation can be inhibited by preventing
mitochondrial depolarization. _J. Mol. Cell. Cardiol._ 48, 673–679 (2010). Article CAS PubMed Google Scholar * Nickel, A. G. _ et al_. Reversal of mitochondrial transhydrogenase causes
oxidative stress in heart failure. _Cell Metab._ 22, 472–484 (2015). Article CAS PubMed Google Scholar * Yusuf, S., Dagenais, G., Pogue, J., Bosch, J. & Sleight, P. Vitamin E
supplementation and cardiovascular events in high-risk patients. Heart Outcomes Prevention Evaluation Study Investigators. _N. Engl. J. Med._ 342, 154–160 (2000). Article CAS PubMed
Google Scholar * Tsujita, K. _ et al_. Effects of edaravone on reperfusion injury in patients with acute myocardial infarction. _Am. J. Cardiol._ 94, 481–484 (2004). Article CAS PubMed
Google Scholar * Escobales, N. _ et al_. Mitochondria-targeted ROS scavenger improves post-ischemic recovery of cardiac function and attenuates mitochondrial abnormalities in aged rats. _J.
Mol. Cell. Cardiol._ 77, 136–146 (2014). Article CAS PubMed PubMed Central Google Scholar * Javadov, S. _ et al_. Mitochondria-targeted antioxidant preserves contractile properties and
mitochondrial function of skeletal muscle in aged rats. _Oncotarget_ 6, 39469–39481 (2015). Article PubMed PubMed Central Google Scholar * Dikalova, A. E. _ et al_. Therapeutic
targeting of mitochondrial superoxide in hypertension. _Circ. Res._ 107, 106–116 (2010). Article CAS PubMed PubMed Central Google Scholar * Liang, H. L., Sedlic, F., Bosnjak, Z. &
Nilakantan, V. SOD1 and MitoTEMPO partially prevent mitochondrial permeability transition pore opening, necrosis, and mitochondrial apoptosis after ATP depletion recovery. _Free Radic. Biol.
Med._ 49, 1550–1560 (2010). Article CAS PubMed Google Scholar * Koyama, H. _ et al_. Antioxidants improve the phenotypes of dilated cardiomyopathy and muscle fatigue in mitochondrial
superoxide dismutase-deficient mice. _Molecules_ 18, 1383–1393 (2013). Article CAS PubMed PubMed Central Google Scholar * Kawakami, S. _ et al_. Antioxidant, EUK-8, prevents murine
dilated cardiomyopathy. _Circ. J._ 73, 2125–2134 (2009). Article CAS PubMed Google Scholar * van Empel, V. P. _ et al_. EUK-8, a superoxide dismutase and catalase mimetic, reduces
cardiac oxidative stress and ameliorates pressure overload-induced heart failure in the harlequin mouse mutant. _J. Am. Coll. Cardiol._ 48, 824–832 (2006). Article CAS PubMed Google
Scholar * Rosca, M., Minkler, P. & Hoppel, C. L. Cardiac mitochondria in heart failure: normal cardiolipin profile and increased threonine phosphorylation of complex IV. _Biochim.
Biophys. Acta_ 1807, 1373–1382 (2011). Article CAS PubMed Google Scholar * Acin-Perez, R. _ et al_. Respiratory complex III is required to maintain complex I in mammalian mitochondria.
_Mol. Cell_ 13, 805–815 (2004). Article CAS PubMed PubMed Central Google Scholar * Acin-Perez, R., Fernandez-Silva, P., Peleato, M. L., Perez-Martos, A. & Enriquez, J. A.
Respiratory active mitochondrial supercomplexes. _Mol. Cell_ 32, 529–539 (2008). Article CAS PubMed Google Scholar * Lapuente-Brun, E. _ et al_. Supercomplex assembly determines electron
flux in the mitochondrial electron transport chain. _Science_ 340, 1567–1570 (2013). Article CAS PubMed Google Scholar * Maranzana, E., Barbero, G., Falasca, A. I., Lenaz, G. &
Genova, M. L. Mitochondrial respiratory supercomplex association limits production of reactive oxygen species from complex I. _Antioxid. Redox Signal._ 19, 1469–1480 (2013). Article CAS
PubMed PubMed Central Google Scholar * Rosca, M. G. _ et al_. Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. _Cardiovasc. Res._ 80, 30–39
(2008). Article CAS PubMed PubMed Central Google Scholar * Nicholls, D. G. & Ferguson, S. J. _Bioenergetics_ 4th edn (Academic, 2013). Google Scholar * Chouchani, E. T. _ et al_.
Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. _Nature_ 515, 431–435 (2014). Article CAS PubMed PubMed Central Google Scholar * Okonko, D. O.
& Shah, A. M. Heart failure: mitochondrial dysfunction and oxidative stress in CHF. _Nat. Rev. Cardiol._ 12, 6–8 (2015). Article CAS PubMed Google Scholar * Molyneux, S. L. _ et
al_. Coenzyme Q10: an independent predictor of mortality in chronic heart failure. _J. Am. Coll. Cardiol._ 52, 1435–1441 (2008). Article CAS PubMed Google Scholar * McMurray, J. J. _ et
al_. Coenzyme Q10, rosuvastatin, and clinical outcomes in heart failure: a pre-specified substudy of CORONA (controlled rosuvastatin multinational study in heart failure). _J. Am. Coll.
Cardiol._ 56, 1196–1204 (2010). Article CAS PubMed Google Scholar * Mortensen, S. A. _ et al_. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results
from Q-SYMBIO: a randomized double-blind trial. _JACC Heart Fail._ 2, 641–649 (2014). Article PubMed Google Scholar * Murphy, M. P. Targeting lipophilic cations to mitochondria. _Biochim.
Biophys. Acta_ 1777, 1028–1031 (2008). Article CAS PubMed Google Scholar * Smith, R. A. _ et al_. Mitochondria-targeted antioxidants in the treatment of disease. _Ann. NY Acad. Sci._
1147, 105–111 (2008). Article CAS PubMed Google Scholar * Skulachev, V. P. _ et al_. An attempt to prevent senescence: a mitochondrial approach. _Biochim. Biophys. Acta_ 1787, 437–461
(2009). Article CAS PubMed Google Scholar * Graham, D. _ et al_. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy.
_Hypertension_ 54, 322–328 (2009). Article CAS PubMed Google Scholar * Lyamzaev, K. G. _ et al_. Novel mitochondria-targeted antioxidants: plastoquinone conjugated with cationic plant
alkaloids berberine and palmatine. _Pharm. Res._ 28, 2883–2895 (2011). Article CAS PubMed Google Scholar * Enns, G. M. Treatment of mitochondrial disorders: antioxidants and beyond. _J.
Child Neurol._ 29, 1235–1240 (2014). Article PubMed Google Scholar * Jaber, S. & Polster, B. M. Idebenone and neuroprotection: antioxidant, pro-oxidant, or electron carrier? _J.
Bioenerg. Biomembr._ 47, 111–118 (2015). Article CAS PubMed Google Scholar * Sadun, A. A. _ et al_. Effect of EPI-743 on the clinical course of the mitochondrial disease Leber hereditary
optic neuropathy. _Arch. Neurol._ 69, 331–338 (2012). Article PubMed Google Scholar * Lerman-Sagie, T. _ et al_. Dramatic improvement in mitochondrial cardiomyopathy following treatment
with idebenone. _J. Inherit Metab. Dis._ 24, 28–34 (2001). Article CAS PubMed Google Scholar * Chatfield, K. C. _ et al_. Dysregulation of cardiolipin biosynthesis in pediatric heart
failure. _J. Mol. Cell. Cardiol._ 74, 251–259 (2014). Article CAS PubMed PubMed Central Google Scholar * Saini-Chohan, H. K. _ et al_. Cardiolipin biosynthesis and remodeling enzymes
are altered during development of heart failure. _J. Lipid Res._ 50, 1600–1608 (2009). Article CAS PubMed PubMed Central Google Scholar * Sparagna, G. C. & Lesnefsky, E. J.
Cardiolipin remodeling in the heart. _J. Cardiovasc. Pharmacol._ 53, 290–301 (2009). Article CAS PubMed Google Scholar * Chicco, A. J. & Sparagna, G. C. Role of cardiolipin
alterations in mitochondrial dysfunction and disease. _Am. J. Physiol. Cell Physiol._ 292, C33–C44 (2007). Article CAS PubMed Google Scholar * Pfeiffer, K. _ et al_. Cardiolipin
stabilizes respiratory chain supercomplexes. _J. Biol. Chem._ 278, 52873–52880 (2003). Article CAS PubMed Google Scholar * Schagger, H. Respiratory chain supercomplexes of mitochondria
and bacteria. _Biochim. Biophys. Acta_ 1555, 154–159 (2002). Article CAS PubMed Google Scholar * Zhang, M., Mileykovskaya, E. & Dowhan, W. Gluing the respiratory chain together.
Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. _J. Biol. Chem._ 277, 43553–43556 (2002). Article CAS PubMed Google Scholar * Szeto, H. H. &
Birk, A. V. Serendipity and the discovery of novel compounds that restore mitochondrial plasticity. _Clin. Pharmacol. Ther._ 96, 672–683 (2014). Article CAS PubMed Google Scholar *
Szeto, H. H. & Schiller, P. W. Novel therapies targeting inner mitochondrial membrane — from discovery to clinical development. _Pharm. Res._ 28, 2669–2671 (2011). Article CAS PubMed
Google Scholar * Szeto, H. H. Mitochondria-targeted cytoprotective peptides for ischemia−reperfusion injury. _Antioxid. Redox Signal._ 10, 601–619 (2008). Article CAS PubMed Google
Scholar * Sloan, R. C. _ et al_. Mitochondrial permeability transition in the diabetic heart: contributions of thiol redox state and mitochondrial calcium to augmented reperfusion injury.
_J. Mol. Cell. Cardiol._ 52, 1009–1018 (2012). Article CAS PubMed Google Scholar * Kloner, R. A. _ et al_. Reduction of ischemia/reperfusion injury with bendavia, a
mitochondria-targeting cytoprotective peptide. _J. Am. Heart Assoc._ 1, e001644 (2012). Article PubMed PubMed Central CAS Google Scholar * Szeto, H. H. _ et al_. Mitochondria-targeted
peptide accelerates ATP recovery and reduces ischemic kidney injury. _J. Am. Soc. Nephrol._ 22, 1041–1052 (2011). Article CAS PubMed PubMed Central Google Scholar * Siegel, M. P. _ et
al_. Mitochondrial-targeted peptide rapidly improves mitochondrial energetics and skeletal muscle performance in aged mice. _Aging Cell_ 12, 763–771 (2013). Article CAS PubMed Google
Scholar * Brown, D. A. _ et al_. Reduction of early reperfusion injury with the mitochondria-targeting peptide bendavia. _J. Cardiovasc. Pharmacol. Ther._ 19, 121–132 (2013). Article
PubMed PubMed Central CAS Google Scholar * Birk, A. V., Chao, W. M., Bracken, C., Warren, J. D. & Szeto, H. H. Targeting mitochondrial cardiolipin and the cytochrome _c_/cardiolipin
complex to promote electron transport and optimize mitochondrial ATP synthesis. _Br. J. Pharmacol._ 171, 2017–2028 (2013). Article CAS Google Scholar * Birk, A. V. _ et al_. The
mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. _J. Am. Soc. Nephrol._ 24, 1250–1261 (2013). Article CAS PubMed PubMed Central
Google Scholar * Dai, D. F. _ et al_. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. _J. Am. Coll. Cardiol._ 58, 73–82 (2011). Article CAS PubMed
PubMed Central Google Scholar * Dai, D. F. _ et al_. Global proteomics and pathway analysis of pressure-overload-induced heart failure and its attenuation by mitochondrial-targeted
peptides. _Circ. Heart Fail._ 6, 1067–1076 (2013). Article CAS PubMed Google Scholar * Shi, J. _ et al_. Bendavia restores mitochondrial energy metabolism gene expression and suppresses
cardiac fibrosis in the border zone of the infarcted heart. _Life Sci._ 141, 170–178 (2015). Article CAS PubMed PubMed Central Google Scholar * Andersson, D. C. _ et al_. Mitochondrial
production of reactive oxygen species contributes to the β-adrenergic stimulation of mouse cardiomycytes. _J. Physiol._ 589, 1791–1801 (2011). Article CAS PubMed PubMed Central Google
Scholar * Eirin, A. _ et al_. Mitochondrial targeted peptides attenuate residual myocardial damage after reversal of experimental renovascular hypertension. _J. Hypertens._ 32, 154–165
(2014). Article CAS PubMed PubMed Central Google Scholar * Sabbah, H. N. _ et al_. Chronic therapy with elamipretide (MTP-131), a novel mitochondria-targeting peptide, improves left
ventricular and mitochondrial function in dogs with advanced heart failure. _Circ. Heart Fail._ 9, e002206 (2016). Article CAS PubMed PubMed Central Google Scholar * Min, K. _ et al_.
Mitochondrial-targeted antioxidants protect skeletal muscle against immobilization-induced muscle atrophy. _J. Appl. Physiol._ 111, 1459–1466 (2011). Article CAS PubMed PubMed Central
Google Scholar * Powers, S. K. _ et al_. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. _Crit. Care Med._ 39, 1749–1759 (2011).
Article CAS PubMed PubMed Central Google Scholar * Halestrap, A. P., Clarke, S. J. & Javadov, S. A. Mitochondrial permeability transition pore opening during myocardial reperfusion
— a target for cardioprotection. _Cardiovasc. Res._ 61, 372–385 (2004). Article CAS PubMed Google Scholar * Halestrap, A. P. & Pasdois, P. The role of the mitochondrial permeability
transition pore in heart disease. _Biochim. Biophys. Acta_ 1787, 1402–1415 (2009). Article CAS PubMed Google Scholar * Kowaltowski, A. J., Castilho, R. F. & Vercesi, A. E.
Mitochondrial permeability transition and oxidative stress. _FEBS Lett._ 495, 12–15 (2001). Article CAS PubMed Google Scholar * Sharov, V. G., Todor, A., Khanal, S., Imai, M. &
Sabbah, H. N. Cyclosporine A attenuates mitochondrial permeability transition and improves mitochondrial respiratory function in cardiomyocytes isolated from dogs with heart failure. _J.
Mol. Cell. Cardiol._ 42, 150–158 (2007). Article CAS PubMed Google Scholar * Sharov, V. G., Todor, A. V., Imai, M. & Sabbah, H. N. Inhibition of mitochondrial permeability transition
pores by cyclosporine A improves cytochrome _c_ oxidase function and increases rate of ATP synthesis in failing cardiomyocytes. _Heart Fail. Rev._ 10, 305–310 (2005). Article CAS PubMed
Google Scholar * Lu, X., Kwong, J. Q., Molkentin, J. D. & Bers, D. M. Individual cardiac mitochondria undergo rare transient permeability transition pore openings. _Circ. Res._ 118,
834–841 (2016). Article CAS PubMed Google Scholar * Baines, C. P. The molecular composition of the mitochondrial permeability transition pore. _J. Mol. Cell. Cardiol._ 46, 850–857
(2009). Article CAS PubMed PubMed Central Google Scholar * Halestrap, A. P. What is the mitochondrial permeability transition pore? _J. Mol. Cell. Cardiol._ 46, 821–831 (2009). Article
CAS PubMed Google Scholar * Baines, C. P. _ et al_. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. _Nature_ 434, 658–662 (2005).
Article CAS PubMed Google Scholar * Baines, C. P., Kaiser, R. A., Sheiko, T., Craigen, W. J. & Molkentin, J. D. Voltage-dependent anion channels are dispensable for
mitochondrial-dependent cell death. _Nat. Cell Biol._ 9, 550–555 (2007). Article CAS PubMed PubMed Central Google Scholar * Kokoszka, J. E. _ et al_. The ADP/ATP translocator is not
essential for the mitochondrial permeability transition pore. _Nature_ 427, 461–465 (2004). Article CAS PubMed PubMed Central Google Scholar * Giorgio, V. _ et al_. Dimers of
mitochondrial ATP synthase form the permeability transition pore. _Proc. Natl Acad. Sci. USA_ 110, 5887–5892 (2013). Article CAS PubMed PubMed Central Google Scholar * Carraro, M. _ et
al_. Channel formation by yeast F-ATP synthase and the role of dimerization in the mitochondrial permeability transition. _J. Biol. Chem._ 289, 15980–15985 (2014). Article CAS PubMed
PubMed Central Google Scholar * Ghaffari, S., Kazemi, B., Toluey, M. & Sepehrvand, N. The effect of prethrombolytic cyclosporine-A injection on clinical outcome of acute anterior
ST-elevation myocardial infarction. _Cardiovasc. Ther._ 31, e34–e39 (2013). Article CAS PubMed Google Scholar * MITOCARE Study Group. Rationale and design of the 'MITOCARE'
Study: a phase II, multicenter, randomized, double-blind, placebo-controlled study to assess the safety and efficacy of TRO40303 for the reduction of reperfusion injury in patients
undergoing percutaneous coronary intervention for acute myocardial infarction. _Cardiology_ 123, 201–207 (2012). Article CAS Google Scholar * Mewton, N. _ et al_. Effect of cyclosporine
on left ventricular remodeling after reperfused myocardial infarction. _J. Am. Coll. Cardiol._ 55, 1200–1205 (2010). Article CAS PubMed Google Scholar * Piot, C. _ et al_. Effect of
cyclosporine on reperfusion injury in acute myocardial infarction. _N. Engl. J. Med._ 359, 473–481 (2008). Article CAS PubMed Google Scholar * Naesens, M., Kuypers, D. R. & Sarwal,
M. Calcineurin inhibitor nephrotoxicity. _Clin. J. Am. Soc. Nephrol._ 4, 481–508 (2009). Article CAS PubMed Google Scholar * Tabara, L. C. _ et al_. Mitochondria-targeted therapies for
acute kidney injury. _Expert Rev. Mol. Med._ 16, e13 (2014). Article PubMed CAS Google Scholar * Hiemstra, J. A. _ et al_. A new twist on an old idea part 2: cyclosporine preserves
normal mitochondrial but not cardiomyocyte function in mini-swine with compensated heart failure. _Physiol. Rep._ 2, e12050 (2014). Article PubMed PubMed Central CAS Google Scholar *
Bayeva, M., Sawicki, K. T., Butler, J., Gheorghiade, M. & Ardehali, H. Molecular and cellular basis of viable dysfunctional myocardium. _Circ. Heart Fail._ 7, 680–691 (2014). Article
PubMed PubMed Central Google Scholar * Sohn, Y. S., Breuer, W., Munnich, A. & Cabantchik, Z. I. Redistribution of accumulated cell iron: a modality of chelation with therapeutic
implications. _Blood_ 111, 1690–1699 (2008). Article CAS PubMed Google Scholar * O'Rourke, B. _ et al_. Mechanisms of altered excitation−contraction coupling in canine
tachycardia-induced heart failure, I: experimental studies. _Circ. Res._ 84, 562–570 (1999). Article CAS PubMed Google Scholar * Wilson, L. D. _ et al_. Heart failure enhances
susceptibility to arrhythmogenic cardiac alternans. _Heart Rhythm_ 6, 251–259 (2009). Article PubMed Google Scholar * Brown, D. A. & Cascio, W. E. 'Leaky' ryanodine
receptors and sudden cardiac death. _Cardiovasc. Res._ 84, 343–344 (2009). Article CAS PubMed Google Scholar * Brown, D. A. & O'Rourke, B. Cardiac mitochondria and arrhythmias.
_Cardiovasc. Res._ 88, 241–249 (2010). Article CAS PubMed PubMed Central Google Scholar * Kosmala, W. _ et al_. Effect of _I_f-channel inhibition on hemodynamic status and exercise
tolerance in heart failure with preserved ejection fraction: a randomized trial. _J. Am. Coll. Cardiol._ 62, 1330–1338 (2013). Article CAS PubMed Google Scholar * Gorski, P. A.,
Ceholski, D. K. & Hajjar, R. J. Altered myocardial calcium cycling and energetics in heart failure — a rational approach for disease treatment. _Cell. Metab._ 21, 183–194 (2015). Article
CAS PubMed PubMed Central Google Scholar * Kawase, Y. & Hajjar, R. J. The cardiac sarcoplasmic/endoplasmic reticulum calcium ATPase: a potent target for cardiovascular diseases.
_Nat. Clin. Pract. Cardiovasc. Med._ 5, 554–565 (2008). Article CAS PubMed Google Scholar * Kho, C., Lee, A. & Hajjar, R. J. Altered sarcoplasmic reticulum calcium cycling — targets
for heart failure therapy. _Nat. Rev. Cardiol._ 9, 717–733 (2012). Article CAS PubMed PubMed Central Google Scholar * Gong, H. B., Wang, L., Lv, Q. & Wang, J. Improved systolic
function of rat cardiocytes during heart failure by overexpression of SERCA2a. _Eur. Rev. Med. Pharmacol. Sci._ 20, 1590–1596 (2016). PubMed Google Scholar * Mattila, M., Koskenvuo, J.,
Soderstrom, M., Eerola, K. & Savontaus, M. Intramyocardial injection of SERCA2a-expressing lentivirus improves myocardial function in doxorubicin-induced heart failure. _J. Gene Med._
18, 124–133 (2016). Article CAS PubMed Google Scholar * Greenberg, B. _ et al_. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease
(CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. _Lancet_ 387, 1178–1186 (2016). Article CAS PubMed Google Scholar * Belevych, A. E. _ et al_.
Redox modification of ryanodine receptors underlies calcium alternans in a canine model of sudden cardiac death. _Cardiovasc. Res._ 84, 387–395 (2009). Article CAS PubMed PubMed Central
Google Scholar * Despa, S., Islam, M. A., Weber, C. R., Pogwizd, S. M. & Bers, D. M. Intracellular Na+ concentration is elevated in heart failure but Na/K pump function is unchanged.
_Circulation_ 105, 2543–2548 (2002). Article CAS PubMed Google Scholar * Pieske, B. & Houser, S. R. [Na+]i handling in the failing human heart. _Cardiovasc. Res._ 57, 874–886 (2003).
Article CAS PubMed Google Scholar * Pieske, B., Houser, S. R., Hasenfuss, G. & Bers, D. M. Sodium and the heart: a hidden key factor in cardiac regulation. _Cardiovasc. Res._ 57,
871–872 (2003). Article CAS PubMed Google Scholar * Pieske, B. _ et al_. Rate dependence of [Na+]i and contractility in nonfailing and failing human myocardium. _Circulation_ 106,
447–453 (2002). Article CAS PubMed Google Scholar * Griffiths, E. J. Mitochondrial calcium transport in the heart: physiological and pathological roles. _J. Mol. Cell. Cardiol._ 46,
789–803 (2009). Article CAS PubMed Google Scholar * Maack, C. _ et al_. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs
energetic adaptation in cardiac myocytes. _Circ. Res._ 99, 172–182 (2006). Article CAS PubMed PubMed Central Google Scholar * Palty, R. _ et al_. NCLX is an essential component of
mitochondrial Na+/Ca2+ exchange. _Proc. Natl Acad. Sci. USA_ 107, 436–441 (2010). Article CAS PubMed Google Scholar * Nicolau, S. M. _ et al_. Mitochondrial Na+/Ca2+-exchanger blocker
CGP37157 protects against chromaffin cell death elicited by veratridine. _J. Pharmacol. Exp. Ther._ 330, 844–854 (2009). Article CAS PubMed Google Scholar * Liu, T., Brown, D. A. &
O'Rourke, B. Role of mitochondrial dysfunction in cardiac glycoside toxicity. _J. Mol. Cell. Cardiol._ 49, 728–736 (2010). Article CAS PubMed PubMed Central Google Scholar *
Primessnig, U. _ et al_. Novel pathomechanisms of cardiomyocyte dysfunction in a model of heart failure with preserved ejection fraction. _Eur. J. Heart Fail._ 18, 987–997 (2016). Article
CAS PubMed Google Scholar * Mamidi, R., Gresham, K. S., Li, A., dos Remedios, C. G. & Stelzer, J. E. Molecular effects of the myosin activator omecamtiv mecarbil on contractile
properties of skinned myocardium lacking cardiac myosin binding protein-C. _J. Mol. Cell. Cardiol._ 85, 262–272 (2015). Article CAS PubMed PubMed Central Google Scholar * Malik, F. I. _
et al_. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. _Science_ 331, 1439–1443 (2011). Article CAS PubMed PubMed Central Google Scholar *
Cleland, J. G. _ et al_. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: a double-blind, placebo-controlled, crossover,
dose-ranging phase 2 trial. _Lancet_ 378, 676–683 (2011). Article CAS PubMed Google Scholar * Greenberg, B. H. _ et al_. Safety and tolerability of omecamtiv mecarbil during exercise in
patients with ischemic cardiomyopathy and angina. _JACC Heart Fail._ 3, 22–29 (2015). Article PubMed Google Scholar * Teerlink, J. R. _ et al_. Acute treatment with omecamtiv mecarbil to
increase contractility in acute heart failure: the ATOMIC-AHF study. _J. Am. Coll. Cardiol._ 67, 1444–1455 (2016). Article CAS PubMed Google Scholar * Teerlink, J. R. _ et al_. Chronic
oral study of myosin activation to increase contractility in heart failure (COSMIC-HF): final results from a double-blind, randomized, placebo-controlled, multicenter study [abstract].
Presented at American Heart Association Scientific Sessions 2015. * Moin, D. S., Sackheim, J., Hamo, C. E. & Butler, J. Cardiac myosin activators in systolic heart failure: more friend
than foe? _Curr. Cardiol. Rep._ 18, 100 (2016). Article PubMed Google Scholar * Bakkehaug, J. P. _ et al_. Myosin activator omecamtiv mecarbil increases myocardial oxygen consumption and
impairs cardiac efficiency mediated by resting myosin ATPase activity. _Circ. Heart Fail._ 8, 766–775 (2015). Article CAS PubMed Google Scholar * Nagy, L. _ et al_. The novel cardiac
myosin activator omecamtiv mecarbil increases the calcium sensitivity of force production in isolated cardiomyocytes and skeletal muscle fibres of the rat. _Br. J. Pharmacol._
http://dx.doi.org/10.1111/bph.13235 (2015). * Phan, T. T. _ et al_. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction
of the left ventricle on exercise and associated with myocardial energy deficiency. _J. Am. Coll. Cardiol._ 54, 402–409 (2009). Article PubMed Google Scholar * Esposito, A. _ et al_.
Impaired left ventricular energy metabolism in patients with hypertrophic cardiomyopathy is related to the extension of fibrosis at delayed gadolinium-enhanced magnetic resonance imaging.
_Heart_ 95, 228–233 (2009). Article CAS PubMed Google Scholar * Smith, C. S., Bottomley, P. A., Schulman, S. P., Gerstenblith, G. & Weiss, R. G. Altered creatine kinase adenosine
triphosphate kinetics in failing hypertrophied human myocardium. _Circulation_ 114, 1151–1158 (2006). Article CAS PubMed PubMed Central Google Scholar * Weiss, R. G., Gerstenblith, G.
& Bottomley, P. A. ATP flux through creatine kinase in the normal, stressed, and failing human heart. _Proc. Natl Acad. Sci. USA_ 102, 808–813 (2005). Article CAS PubMed PubMed
Central Google Scholar * Crilley, J. G. _ et al_. Hypertrophic cardiomyopathy due to sarcomeric gene mutations is characterized by impaired energy metabolism irrespective of the degree of
hypertrophy. _J. Am. Coll. Cardiol._ 41, 1776–1782 (2003). Article CAS PubMed Google Scholar * Beer, M. _ et al_. Absolute concentrations of high-energy phosphate metabolites in normal,
hypertrophied, and failing human myocardium measured noninvasively with 31P-SLOOP magnetic resonance spectroscopy. _J. Am. Coll. Cardiol._ 40, 1267–1274 (2002). Article CAS PubMed Google
Scholar * Beer, M. _ et al_. [Cardiac energy metabolism in heart valve diseases with 31P MR spectroscopy [German]. _Radiologe_ 40, 162–167 (2000). Article CAS PubMed Google Scholar *
Lamb, H. J. _ et al_. Diastolic dysfunction in hypertensive heart disease is associated with altered myocardial metabolism. _Circulation_ 99, 2261–2267 (1999). Article CAS PubMed Google
Scholar * Conway, M. A., Bottomley, P. A., Ouwerkerk, R., Radda, G. K. & Rajagopalan, B. Mitral regurgitation: impaired systolic function, eccentric hypertrophy, and increased severity
are linked to lower phosphocreatine/ATP ratios in humans. _Circulation_ 97, 1716–1723 (1998). Article CAS PubMed Google Scholar * Jung, W. I. _ et al_. 31P NMR spectroscopy detects
metabolic abnormalities in asymptomatic patients with hypertrophic cardiomyopathy. _Circulation_ 97, 2536–2542 (1998). Article CAS PubMed Google Scholar * Starling, R. C., Hammer, D. F.
& Altschuld, R. A. Human myocardial ATP content and _in vivo_ contractile function. _Mol. Cell. Biochem._ 180, 171–177 (1998). Article CAS PubMed Google Scholar * Neubauer, S. _ et
al_. Cardiac high-energy phosphate metabolism in patients with aortic valve disease assessed by 31P-magnetic resonance spectroscopy. _J. Investig. Med._ 45, 453–462 (1997). CAS PubMed
Google Scholar * Neubauer, S. _ et al_. Contributions of 31P-magnetic resonance spectroscopy to the understanding of dilated heart muscle disease. _Eur. Heart J._ 16 (Suppl. O), 115–118
(1995). Article CAS PubMed Google Scholar * Yabe, T., Mitsunami, K., Inubushi, T. & Kinoshita, M. Quantitative measurements of cardiac phosphorus metabolites in coronary artery
disease by 31P magnetic resonance spectroscopy. _Circulation_ 92, 15–23 (1995). Article CAS PubMed Google Scholar * Yabe, T. _ et al_. Detection of myocardial ischemia by 31P magnetic
resonance spectroscopy during handgrip exercise. _Circulation_ 89, 1709–1716 (1994). Article CAS PubMed Google Scholar * Sakuma, H. _ et al_. 31P MR spectroscopy in hypertrophic
cardiomyopathy: comparison with Tl-201 myocardial perfusion imaging. _Am. Heart J._ 125, 1323–1328 (1993). Article CAS PubMed Google Scholar * Schaefer, S. _ et al_. Metabolic response
of the human heart to inotropic stimulation: _in vivo_ phosphorus-31 studies of normal and cardiomyopathic myocardium. _Magn. Reson. Med._ 25, 260–272 (1992). Article CAS PubMed Google
Scholar * de Roos, A. _ et al_. Cardiac metabolism in patients with dilated and hypertrophic cardiomyopathy: assessment with proton-decoupled P-31 MR spectroscopy. _J. Magn. Reson. Imaging_
2, 711–719 (1992). Article CAS PubMed Google Scholar * Neubauer, S. _ et al_. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac
high-energy phosphate metabolism in heart failure. _Circulation_ 86, 1810–1818 (1992). Article CAS PubMed Google Scholar * Regitz, V. & Fleck, E. Myocardial adenine nucleotide
concentrations and myocardial norepinephrine content in patients with heart failure secondary to idiopathic dilated or ischemic cardiomyopathy. _Am. J. Cardiol._ 69, 1574–1580 (1992).
Article CAS PubMed Google Scholar * Conway, M. A. _ et al_. Detection of low phosphocreatine to ATP ratio in failing hypertrophied human myocardium by 31P magnetic resonance
spectroscopy. _Lancet_ 338, 973–976 (1991). Article CAS PubMed Google Scholar * Hardy, C. J., Weiss, R. G., Bottomley, P. A. & Gerstenblith, G. Altered myocardial high-energy
phosphate metabolites in patients with dilated cardiomyopathy. _Am. Heart J._ 122, 795–801 (1991). Article CAS PubMed Google Scholar * Schaefer, S. _ et al_. _In vivo_ phosphorus-31
spectroscopic imaging in patients with global myocardial disease. _Am. J. Cardiol._ 65, 1154–1161 (1990). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS The
roundtable discussion in Stresa, Italy, was organized by Logica Med LLC and funded by an unrestricted grant from Stealth BioTherapeutics. We thank Fumiko Inoue (Logica Med) for her help in
organizing the roundtable meeting. The authors also acknowledge BioCentric, Inc. for their assistance with developing previous versions of the manuscript Figures. D.A.B. has received
research grants from the NIH (NHLBI R01 HL123647 and R15 HL122922) and Stealth BioTherapeutics. B.L.S. is supported by research grants from the NIH (NIA R01 AG049762, NHLBI R01 HL131458,
R01HL126928, and R01HL107715) and Stealth BioTherapeutics. S.R.S. is supported by grants from the NIH (R01 HL123647, R15 HL122922, and R01 AT008375). J.B.has received research support from
the NIH and the European Union. A.A.V. is supported by a grant from the European Commission (FP7-242209-BIOSTAT-CHF). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Human
Nutrition, Foods, and Exercise, Virginia Tech, 1035 Integrated Life Sciences Building, 1981 Kraft Drive, Blacksburg, 24060, Virginia, USA David A. Brown, Justin B. Perry & Mitchell E.
Allen * Division of Cardiovascular Medicine, Department of Medicine, Henry Ford Hospital, 2799 West Grand Boulevard, Detroit, 48202, Michigan, USA Hani N. Sabbah * Division of Cardiology,
Department of Medicine, University of Colorado Denver, 12700 East 19th Avenue, B139, Aurora, 80045, Colorado, USA Brian L. Stauffer * Department of Biochemistry and Molecular Biology, East
Carolina Diabetes and Obesity Institute, Brody School of Medicine, East Carolina University, 115 Heart Drive, Greenville, 27834, North Carolina, USA Saame Raza Shaikh * National Heart &
Lung Institute, National Institute of Health Research Cardiovascular Biomedical Research Unit, Royal Brompton & Harefield Hospitals, Imperial College, London, UK John G. F. Cleland *
Cardiovascular Medicine Section, Boston University School of Medicine and Boston Medical Center, 88 East Newton Street, C-8, Boston, 02118, Massachusetts, USA Wilson S. Colucci * Division of
Cardiology, Health Sciences Center, T-16 Room 080, SUNY at Stony Brook, 11794, New York, USA Javed Butler * Department of Cardiology, University of Groningen, University Medical Center
Groningen, 9713 GZ, Groningen, Netherlands Adriaan A. Voors * Department of Innovative Clinical Trials, University Medical Centre Göttingen (UMG), Robert-Koch-Straße, Göttingen, D-37075,
Germany Stefan D. Anker * University of Michigan School of Medicine, 1500 East Medical Center Drive, Ann Arbor, 48109, Michigan, USA Bertram Pitt * Department of Cardiology, Charité
University Medicine, Campus Virchow Klinikum, and German Heart Center Berlin, Augustenburger Platz 1, Berlin, 13353, Germany Burkert Pieske * Department of Cardiology, National and
Kopodistrian University of Athens, School of Medicine, Heart Failure Unit, Athens University Hospital Attikon, Rimini 1, Athens, 12462, Greece Gerasimos Filippatos * Division of Cardiology,
Duke University Medical Center, 2301 Erwin Road Suite 7400, Durham, 27705, North Carolina, USA Stephen J. Greene * Center for Cardiovascular Innovation, Northwestern University Feinberg
School of Medicine, 201 East Huron, Galter 3–150, Chicago, 60611, Illinois, USA Mihai Gheorghiade Authors * David A. Brown View author publications You can also search for this author
inPubMed Google Scholar * Justin B. Perry View author publications You can also search for this author inPubMed Google Scholar * Mitchell E. Allen View author publications You can also
search for this author inPubMed Google Scholar * Hani N. Sabbah View author publications You can also search for this author inPubMed Google Scholar * Brian L. Stauffer View author
publications You can also search for this author inPubMed Google Scholar * Saame Raza Shaikh View author publications You can also search for this author inPubMed Google Scholar * John G. F.
Cleland View author publications You can also search for this author inPubMed Google Scholar * Wilson S. Colucci View author publications You can also search for this author inPubMed Google
Scholar * Javed Butler View author publications You can also search for this author inPubMed Google Scholar * Adriaan A. Voors View author publications You can also search for this author
inPubMed Google Scholar * Stefan D. Anker View author publications You can also search for this author inPubMed Google Scholar * Bertram Pitt View author publications You can also search for
this author inPubMed Google Scholar * Burkert Pieske View author publications You can also search for this author inPubMed Google Scholar * Gerasimos Filippatos View author publications You
can also search for this author inPubMed Google Scholar * Stephen J. Greene View author publications You can also search for this author inPubMed Google Scholar * Mihai Gheorghiade View
author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS D.A.B. wrote the manuscript, and all authors reviewed and edited it before submission.
CORRESPONDING AUTHOR Correspondence to Mihai Gheorghiade. ETHICS DECLARATIONS COMPETING INTERESTS D.A.B. has received consulting income from Stealth BioTherapeutics. J.G.F.C. reports
consultation with Amgen, Biotronik, GSK, Medtronic, Novartis, Servier, Sorin, and Stealth BioTherapeutics. W.S.C. is a consultant to BMS, Cardioxyl, Johnson & Johnson, Mast, Merck, and
Novartis. J.B. is a consultant to Amgen, Bayer, Boehringer Ingelheim, Cardiocell, Celladon, Novartis, Relypsa, Trevena, Z Pharma, and Zensun. A.A.V. has received consultancy fees and/or
research grants from Alere, Amgen, Bayer, Boehringer, Cardio3Biosciences, Celladon, GSK, Merck/MSD, Novartis, Servier, Singulex, Sphingotec, Trevena, Vifor, and ZS Pharma. B.Pieske reports
speaker's bureau and/or advisory/steering committee honoraria from Abbott Vascular, AstraZeneca, Bayer Healthcare, Novartis Pharma, Servier, and Stealth BioTherapeutics. M.G. has been a
consultant for Abbott Laboratories, Astellas, AstraZeneca, Bayer HealthCare, CorThera, Cytokinetics, DebioPharm, Errekappa Terapeutici, GlaxoSmithKline, Ikaria, Johnson & Johnson,
Medtronic, Merck, Novartis Pharma, Otsuka Pharmaceuticals, Palatin Technologies, Pericor Therapeutics, Protein Design Laboratories, Sanofi-Aventis, Sigma Tau, Solvay Pharmaceuticals, Takeda
Pharmaceutical, and Trevena Therapeutics. The other authors declare no competing interests. POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR
FIG. 3 POWERPOINT SLIDE FOR FIG. 4 POWERPOINT SLIDE FOR TABLE 1 RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or
other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under
the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Brown, D., Perry, J., Allen, M. _et al._ Mitochondrial function as a therapeutic
target in heart failure. _Nat Rev Cardiol_ 14, 238–250 (2017). https://doi.org/10.1038/nrcardio.2016.203 Download citation * Published: 22 December 2016 * Issue Date: April 2017 * DOI:
https://doi.org/10.1038/nrcardio.2016.203 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative





