- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
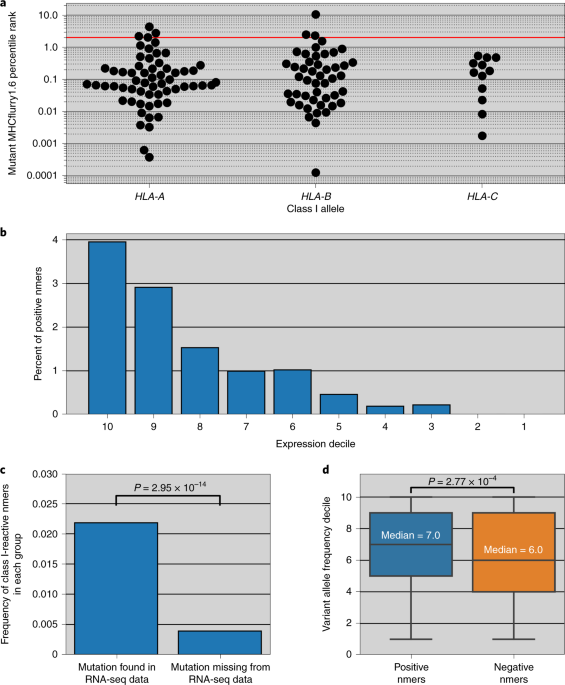
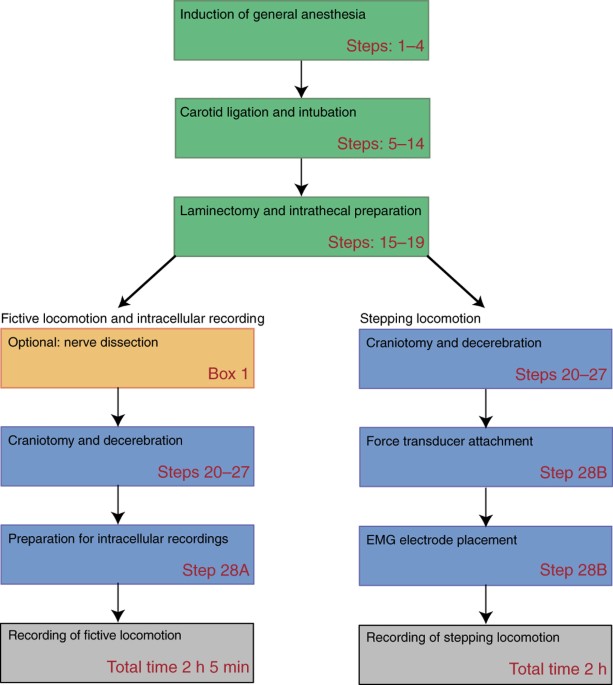
ABSTRACT The adult decerebrate mouse model (a mouse with the cerebrum removed) enables the study of sensory-motor integration and motor output from the spinal cord for several hours without
compromising these functions with anesthesia. For example, the decerebrate mouse is ideal for examining locomotor behavior using intracellular recording approaches, which would not be
possible using current anesthetized preparations. This protocol describes the steps required to achieve a low-blood-loss decerebration in the mouse and approaches for recording signals from
spinal cord neurons with a focus on motoneurons. The protocol also describes an example application for the protocol: the evocation of spontaneous and actively driven stepping, including
optimization of these behaviors in decerebrate mice. The time taken to prepare the animal and perform a decerebration takes ∼2 h, and the mice are viable for up to 3–8 h, which is ample time
to perform most short-term procedures. These protocols can be modified for those interested in cardiovascular or respiratory function in addition to motor function and can be performed by
trainees with some previous experience in animal surgery. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS
OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn
more Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to
full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our
FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS DISENGAGING SPINAL AFFERENT NERVE COMMUNICATION WITH THE BRAIN IN LIVE MICE Article Open access 14 September 2022
DECONSTRUCTING THE MODULAR ORGANIZATION AND REAL-TIME DYNAMICS OF MAMMALIAN SPINAL LOCOMOTOR NETWORKS Article Open access 16 February 2023 LONG-TERM OPTICAL IMAGING OF THE SPINAL CORD IN
AWAKE BEHAVING MICE Article 12 November 2024 REFERENCES * Kiehn, O. Development and functional organization of spinal locomotor circuits. _Curr. Opin. Neurobiol._ 21, 100–109 (2011). Article
CAS Google Scholar * Grossmann, K.S., Giraudin, A., Britz, O., Zhang, J. & Goulding, M. Genetic dissection of rhythmic motor networks in mice. _Prog. Brain Res._ 187, 19–37 (2010).
Article CAS Google Scholar * Whelan, P.J. Shining light into the black box of spinal locomotor networks. _Philos. Trans. R. Soc. Lond. B Biol. Sci._ 365, 2383–2395 (2010). Article Google
Scholar * Dougherty, K.J. et al. Locomotor rhythm generation linked to the output of spinal shox2 excitatory interneurons. _Neuron_ 80, 920–933 (2013). Article CAS Google Scholar *
Lanuza, G.M., Gosgnach, S., Pierani, A., Jessell, T.M. & Goulding, M. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking
movements. _Neuron_ 42, 375–386 (2004). Article CAS Google Scholar * Bretzner, F. & Brownstone, R.M. Lhx3-Chx10 reticulospinal neurons in locomotor circuits. _J. Neurosci._ 33,
14681–14692 (2013). Article CAS Google Scholar * Mendelsohn, A.I., Simon, C.M., Abbott, L.F., Mentis, G.Z. & Jessell, T.M. Activity regulates the incidence of heteronymous
sensory-motor connections. _Neuron_ 87, 111–123 (2015). Article CAS Google Scholar * Hägglund, M. et al. Optogenetic dissection reveals multiple rhythmogenic modules underlying
locomotion. _Proc. Natl. Acad. Sci. USA_ 110, 11589–11594 (2013). Article Google Scholar * Wyart, C. et al. Optogenetic dissection of a behavioural module in the vertebrate spinal cord.
_Nature_ 461, 407–410 (2009). Article CAS Google Scholar * Goulding, M., Bourane, S., Garcia-Campmany, L., Dalet, A. & Koch, S. Inhibition downunder: an update from the spinal cord.
_Curr. Opin. Neurobiol._ 26, 161–166 (2014). Article CAS Google Scholar * Akay, T., Tourtellotte, W.G., Arber, S. & Jessell, T.M. Degradation of mouse locomotor pattern in the absence
of proprioceptive sensory feedback. _Proc. Natl. Acad. Sci. USA_ 111, 16877–16882 (2014). Article CAS Google Scholar * Whelan, P.J. Developmental aspects of spinal locomotor function:
insights from using the _in vitro_ mouse spinal cord preparation. _J. Physiol._ 553, 695–706 (2003). Article CAS Google Scholar * Schmidt, B.J. & Jordan, L.M. The role of serotonin in
reflex modulation and locomotor rhythm production in the mammalian spinal cord. _Brain Res. Bull._ 53, 689–710 (2000). Article CAS Google Scholar * Mentis, G.Z., Alvarez, F.J., Shneider,
N.A., Siembab, V.C. & O'Donovan, M.J. Mechanisms regulating the specificity and strength of muscle afferent inputs in the spinal cord. _Ann. N. Y. Acad. Sci._ 1198, 220–230 (2010).
Article CAS Google Scholar * Mentis, G.Z., Siembab, V.C., Zerda, R., O'Donovan, M.J. & Alvarez, F.J. Primary afferent synapses on developing and adult Renshaw cells. _J.
Neurosci._ 26, 13297–13310 (2006). Article CAS Google Scholar * Meehan, C.F., Sukiasyan, N., Zhang, M., Nielsen, J.B. & Hultborn, H. Intrinsic properties of mouse lumbar motoneurons
revealed by intracellular recording _in vivo_. _J. Neurophysiol._ 103, 2599–2610 (2010). Article CAS Google Scholar * Manuel, M. et al. Fast kinetics, high-frequency oscillations, and
subprimary firing range in adult mouse spinal motoneurons. _J. Neurosci._ 29, 11246–11256 (2009). Article CAS Google Scholar * Meehan, C.F. et al. Intrinsic properties of lumbar motor
neurones in the adult G127insTGGG superoxide dismutase-1 mutant mouse _in vivo_: evidence for increased persistent inward currents. _Acta Physiol._ 200, 361–376 (2010). Article CAS Google
Scholar * Kleiber, M. Body size and metabolic rate. _Physiol. Rev._ 27, 511–541 (1947). Article CAS Google Scholar * Nakanishi, S.T. & Whelan, P.J. A decerebrate adult mouse model
for examining the sensorimotor control of locomotion. _J. Neurophysiol._ 107, 500–515 (2012). Article Google Scholar * Meehan, C.F., Grondahl, L., Nielsen, J.B. & Hultborn, H. Fictive
locomotion in the adult decerebrate and spinal mouse _in vivo_. _J. Physiol._ 590, 289–300 (2012). Article CAS Google Scholar * Iglesias, C. et al. Mixed mode oscillations in mouse spinal
motoneurons arise from a low excitability state. _J. Neurosci._ 31, 5829–5840 (2011). Article CAS Google Scholar * Delestrée, N. et al. Adult spinal motoneurones are not hyperexcitable
in a mouse model of inherited amyotrophic lateral sclerosis. _J. Physiol._ 592, 1687–1703 (2014). Article Google Scholar * Hedegaard, A. et al. Postactivation depression of the Ia EPSP in
motoneurons is reduced in both the G127X SOD1 model of amyotrophic lateral sclerosis and in aged mice. _J. Neurophysiol._ 114, 1196–1210 (2015). Article CAS Google Scholar * Lehnhoff, J.,
Moldovan, M., Hedegaard, L.G. & Meehan, C.F. _In vivo_ intracellular recordings from spinal lumbar motoneurones in P0-deficient mice indicate an activity-dependent axonal conduction
failure in otherwise functional motoneurones. _Proc. Physiol. Soc._ 31, PCA079 (2014). Google Scholar * Alstermark, B. & Ogawa, J. recordings of bulbospinal excitation in adult mouse
forelimb motoneurons. _J. Neurophysiol._ 92, 1958–1962 (2004). Article Google Scholar * Wilson, R.J.A., Chersa, T. & Whelan, P.J. Tissue PO2 and the effects of hypoxia on the
generation of locomotor-like activity in the _in vitro_ spinal cord of the neonatal mouse. _Neuroscience_ 117, 183–196 (2003). Article CAS Google Scholar * Husch, A., Dietz, S.B., Hong,
D.N. & Harris-Warrick, R.M. Adult spinal V2a interneurons show increased excitability and serotonin-dependent bistability. _J. Neurophysiol._ 113, 1124–1134 (2015). Article CAS Google
Scholar * Mitra, P. & Brownstone, R.M. An _in vitro_ spinal cord slice preparation for recording from lumbar motoneurons of the adult mouse. _J. Neurophysiol._ 107, 728–741 (2012).
Article Google Scholar * Husch, A., Cramer, N. & Harris-Warrick, R.M. Long-duration perforated patch recordings from spinal interneurons of adult mice. _J. Neurophysiol._ 106,
2783–2789 (2011). Article Google Scholar * Bennett, D.J., Li, Y. & Siu, M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded _in vitro_. _J. Neurophysiol._
86, 1955–1971 (2001). Article CAS Google Scholar * Long, S.K., Evans, R.H., Cull, L., Krijzer, F. & Bevan, P. An _in vitro_ mature spinal cord preparation from the rat.
_Neuropharmacology_ 27, 541–546 (1988). Article CAS Google Scholar * Jiang, M.C. & Heckman, C.J. _In vitro_ sacral cord preparation and motoneuron recording from adult mice. _J.
Neurosci. Methods_ 156, 31–36 (2006). Article CAS Google Scholar * Silverman, J., Suckow, M.A. & Murthy, S. _The IACUC Handbook_ 3rd edn. (CRC Press, 2014). * Carlin, K.P., Jiang, Z.
& Brownstone, R.M. Characterization of calcium currents in functionally mature mouse spinal motoneurons. _Eur. J. Neurosci._ 12, 1624–1634 (2000). Article CAS Google Scholar *
Heckman, C. & Lee, R. Advances in measuring active dendritic currents in spinal motoneurons. in _Motor Neurobiology of the Spinal Cord_ (ed. Cope, T.C.) 89–105 (CRC Press, 2001). * Lee,
R.H. & Heckman, C.J. Bistability in spinal motoneurons _in vivo_: systematic variations in persistent inward currents. _J. Neurophysiol._ 80, 583–593 (1998). Article CAS Google Scholar
* Jordan, L.M., Liu, J., Hedlund, P.B., Akay, T. & Pearson, K.G. Descending command systems for the initiation of locomotion in mammals. _Brain Res. Rev._ 57, 183–191 (2008). Article
CAS Google Scholar * Conway, B.A., Hultborn, H. & Kiehn, O. Proprioceptive input resets central locomotor rhythm in the spinal cat. _Exp. Brain Res._ 68, 643–656 (1987). Article CAS
Google Scholar * Duysens, J. & Pearson, K.G. Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. _Brain Res._ 187, 321–332 (1980). Article CAS
Google Scholar * Whelan, P.J. Control of locomotion in the decerebrate cat. _Prog. Neurobiol._ 49, 481–515 (1996). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This
work was supported by Natural Sciences and Engineering Research Council grants to P.J.W. M.M. received funds from NIH NINDS R01NS077863. C.F.M. received funds from an EU FP7 Marie Curie
Fellowship and project grants from the Lundbeck Foundation. C.F.M. acknowledges the technical assistance of L. Grøhndahl of the Meehan laboratory, the assistance of A. Hedegaard of the
Meehan laboratory for the voltage clamp experiments, and advice regarding the voltage clamp and the voltage clamp external gain instrument from C.J. Heckman (Northwestern University). K.A.M.
received a studentship from the Branch Out Neurological Foundation and the Hotchkiss Brain Institute. P.J.W. and K.A.M. acknowledge the technical assistance of A. Krajacic of the Whelan
laboratory. AUTHOR INFORMATION Author notes * Claire F Meehan and Kyle A Mayr: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Centre for Neuroscience, University
of Copenhagen, Copenhagen, Denmark Claire F Meehan * Department of Comparative Biology and Experimental Medicine, University of Calgary, Calgary, Alberta, Canada Kyle A Mayr & Patrick J
Whelan * CNRS UMR 8119, Université Paris Descartes, Paris, France Marin Manuel * Department of Biology, University of Hawaii at Hilo, Hilo, Hawaii, USA Stan T Nakanishi Authors * Claire F
Meehan View author publications You can also search for this author inPubMed Google Scholar * Kyle A Mayr View author publications You can also search for this author inPubMed Google Scholar
* Marin Manuel View author publications You can also search for this author inPubMed Google Scholar * Stan T Nakanishi View author publications You can also search for this author inPubMed
Google Scholar * Patrick J Whelan View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS C.F.M., K.A.M., M.M., and S.T.N. performed the
experiments, analyzed the data, and prepared figures. P.J.W. wrote the paper and edited figures. C.F.M., K.A.M., M.M., S.T.N., and P.J.W. conceived of the experiments. C.F.M., K.A.M.,
S.T.N., M.M., and P.J.W. edited the manuscript. CORRESPONDING AUTHOR Correspondence to Patrick J Whelan. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial
interests. INTEGRATED SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1 INTRAMUSCULAR HOOK ELECTRODE FOR RECORDING ELECTROMYOGRAM ACTIVITY WITHIN MUSCLES (A) Rounded and sharpened curved
spatula used for decerebration. (B) Custom made leg holder used to easily make a mineral oil bath for the hindlimb muscles and nerves. (C) One completed intramuscular EMG hook electrode
using 3 stranded Teflon coated wire (A-M systems, cat No.793400) run through the lumen of a 23-gauge needle (B-D precisionGlide IM, cat No.305145). (D) Stripped 2-3 mm of Teflon coating from
the end of the stainless steel wire. (E) 180o bend backwards, creating a hook. (F) Stainless steel wire hook pulled backwards to rest in the lowest part of the bevel of the lumen. A refers
to Step 28 of procedure, b refers to Step 22. C-F refers to Step 32B. SUPPLEMENTARY FIGURE 2 INCREASE IN EMG TONE INDICATING A BOUT OF LOCOMOTION. Increase in the amplitude of flexor and
extensor EMG (tibialis anterior and gastrocnemius respectively), in the decerebrate preparation, indicating that a locomotor bout was imminent and that the treadmill should be turned on.
Tibialis Anterior (TA), Gastrocnemius (Gast). All experiments should be performed in accordance with relevant guidelines and regulations. Local ethics committees have approved all
procedures. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES AND TEXT Supplementary Figures 1 and 2. (PDF 352 kb) SUPPLEMENTARY VIDEO 1. STEPPING BEHAVIOR OF A DECEREBRATE MOUSE OVER A WHEEL.
This video shows expected decerebrate walking activity and illustrates the outcome of intrathecal application of 5-HT. (MP4 5164 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Meehan, C., Mayr, K., Manuel, M. _et al._ Decerebrate mouse model for studies of the spinal cord circuits. _Nat Protoc_ 12, 732–747 (2017).
https://doi.org/10.1038/nprot.2017.001 Download citation * Published: 09 March 2017 * Issue Date: April 2017 * DOI: https://doi.org/10.1038/nprot.2017.001 SHARE THIS ARTICLE Anyone you share
the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative