- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
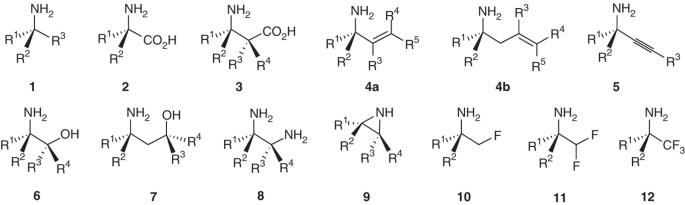
ABSTRACT Chiral amines are prevalent in many bioactive molecules, including amino acids and pharmaceutical agents. _tert_-Butanesulfinamide (tBS) is a chiral amine reagent that has enabled
the reliable asymmetric synthesis of a very broad range of different amine structures from simple, readily available starting materials. Three steps are commonly applied to the asymmetric
synthesis of amines: (i) condensation of tBS with a carbonyl compound, (ii) nucleophile addition and (iii) _tert_-butanesulfinyl group cleavage. Here we demonstrate these steps with the
preparation of a propargylic tertiary carbinamine, one of a class of amines that have been used for many different biological purposes, including click chemistry applications,
diversity-oriented synthesis, the preparation of peptide isosteres and the development of protease inhibitors as drug candidates and imaging agents. The process described here can be
performed in 3–4 d. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution
Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full
article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs *
Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS DEOXYGENATIVE PHOTOCHEMICAL ALKYLATION OF SECONDARY AMIDES ENABLES A STREAMLINED SYNTHESIS OF SUBSTITUTED AMINES Article Open
access 22 January 2025 STEREOCONTROLLED 1,3-NITROGEN MIGRATION TO ACCESS CHIRAL Α-AMINO ACIDS Article 04 April 2022 STEREOSELECTIVE AMINO ALCOHOL SYNTHESIS VIA CHEMOSELECTIVE
ELECTROCATALYTIC RADICAL CROSS-COUPLINGS Article 03 January 2025 REFERENCES * Nugent, T.C. _Chiral Amine Synthesis: Methods, Developments and Applications_ 494 (Wiley, 2010). * Okamoto, Y.
& Ikai, T. Chiral HPLC for efficient resolution of enantiomers. _Chem. Soc. Rev._ 37, 2593–2608 (2008). Article CAS Google Scholar * Noyori, R. Asymmetric catalysis: science and
opportunities (Nobel lecture). _Angew. Chem Intl. Edn._ 41, 2008–2022 (2002). Article CAS Google Scholar * Savile, C.K. et al. Biocatalytic asymmetric synthesis of chiral amines from
ketones applied to sitagliptin manufacture. _Science_ 329, 305–309 (2010). Article CAS Google Scholar * Kroutil, W. et al. Asymmetric preparation of _prim-_, _sec-_, and _tert_-amines
employing selected biocatalysts. _Org. Process Res. Dev._ 17, 751–759 (2013). Article CAS Google Scholar * Friestad, G.K. & Mathies, A.K. Recent developments in asymmetric catalytic
addition to C=N bonds. _Tetrahedron_ 63, 2541–2569 (2007). Article CAS Google Scholar * Kobayashi, S., Mori, Y., Fossey, J.S. & Salter, M.M. Catalytic enantioselective formation of
C-C bonds by addition to imines and hydrazones: a ten-year update. _Chem. Rev._ 111, 2626–2704 (2011). Article CAS Google Scholar * Enders, D. & Reinhold, U. Asymmetric synthesis of
amines by nucleophilic 1,2-addition of organometallic reagents to the CN-double bond. _Tetrahedron Asymmetry_ 8, 1895–1946 (1997). Article CAS Google Scholar * Robak, M.T., Herbage, M.A.
& Ellman, J.A. Synthesis and applications of _tert_-butanesulfinamide. _Chem. Rev._ 110, 3600–3740 (2010). Article CAS Google Scholar * Ferreira, F., Botuha, C., Chemla, F. &
Perez-Luna, A. _tert_-Butanesulfinimines: structure, synthesis and synthetic applications. _Chem. Soc. Rev._ 38, 1162–1186 (2009). Article CAS Google Scholar * Lin, G.-Q., Xu, M.-H.,
Zhong, Y.-W. & Sun, X.-W. An advance on exploring _N_-_tert_-butanesulfinyl imines in asymmetric synthesis of chiral amines. _Acc. Chem. Res._ 41, 831–840 (2008). Article CAS Google
Scholar * Morton, D. & Stockman, R.A. Chiral non-racemic sulfinimines: versatile reagents for asymmetric synthesis. _Tetrahedron_ 62, 8869–8905 (2006). Article CAS Google Scholar *
Senanayake, C.H., Han, Z. & Krishnamurthy, D. Organosulfur chemistry in asymmetric synthesis. (Wiley, 2008). * Senanayake, C.H., Krishnamurthy, D., Lu, Z.-H., Han, Z. & Gallon, E.
Enantiopure sulfoxides and sulfinamides: recent developments in their stereoselective synthesis and application to asymmetric synthesis. _Aldrichim. Acta_ 38, 93–103 (2005). CAS Google
Scholar * Liu, G., Cogan, D.A., Owens, T.D., Tang, T.P. & Ellman, J.A. Synthesis of enantiomerically pure _N_-_tert_-butanesulfinyl imines (_tert_-butanesulfinimines) by the direct
condensation of _tert_-butanesulfinamide with aldehydes and ketones. _J. Org. Chem._ 64, 1278–1284 (1999). Article CAS Google Scholar * Datta, G.K. & Ellman, J.A. Racemization free
protocol for the synthesis of _N_-_tert_-butanesulfinyl ketimines. _J. Org. Chem._ 75, 6283–6285 (2010). Article CAS Google Scholar * Higashibayashi, S., Tohmiya, H., Mori, T., Hashimoto,
K. & Nakata, M. Synthesis of sulfinimines by direct condensation of sulfinamides with aldehydes using Cs2CO3 as an activating and dehydrating reagent. _Synlett_ 2004, 457–460 (2004).
Google Scholar * Huang, Z., Zhang, M., Wang, Y. & Qin, Y. KHSO4-mediated condensation reactions of _tert_-butanesulfinamide with aldehydes. Preparation of _tert_-butanesulfinyl
aldimines. _Synlett_ 2005, 1334–1336 (2005). Article Google Scholar * Collados, J.F., Toledano, E., Guijarro, D. & Yus, M. Microwave-assisted solvent-free synthesis of enantiomerically
pure _N_-(_tert_-butylsulfinyl)imines. _J. Org. Chem._ 77, 5744–5750 (2012). Article CAS Google Scholar * Guijarro, D., Pablo, Ó. & Yus, M. Ruthenium-catalysed asymmetric transfer
hydrogenation of _N_-(_tert_-butanesulfinyl)imines. _Tetrahedron Lett._ 50, 5386–5388 (2009). Article CAS Google Scholar * Weix, D.J., Shi, Y. & Ellman, J.A. Diastereoselective and
enantioselective Rh(I)-catalyzed additions of arylboronic acids to _N_-_tert_-butanesulfinyl and _N_-diphenylphosphinoyl aldimines. _J. Am. Chem. Soc._ 127, 1092–1093 (2005). Article CAS
Google Scholar * Beenen, M.A., Weix, D.J. & Ellman, J.A. Asymmetric synthesis of protected arylglycines by rhodium-catalyzed addition of arylboronic acids to _N_-_tert_-butanesulfinyl
imino esters. _J. Am. Chem. Soc._ 128, 6304–6305 (2006). Article CAS Google Scholar * Dai, H. & Lu, X. Diastereoselective synthesis of arylglycine derivatives by cationic
palladium(II)-catalyzed addition of arylboronic acids to _N_-_tert_-butanesulfinyl imino esters. _Org. Lett._ 9, 3077–3080 (2007). Article CAS Google Scholar * Xiao, X. et al. Selective
diethylzinc reduction of imines in the presence of ketones catalyzed by Ni(acac)2 . _Org. Lett._ 8, 139–142 (2005). Article Google Scholar * Bolshan, Y. & Batey, R.A. A
room-temperature protocol for the Rhodium(I)-catalyzed addition of arylboron compounds to sulfinimines. _Org. Lett._ 7, 1481–1484 (2005). Article CAS Google Scholar * Boebel, T.A. &
Hartwig, J.F. Conversion of 1,3-disubstituted arenes to chiral α,α-diaryl methylammonium chlorides using arene borylation. _Tetrahedron_ 64, 6824–6830 (2008). Article CAS Google Scholar *
Beenen, M.A., An, C. & Ellman, J.A. Asymmetric copper-catalyzed synthesis of α-amino boronate esters from _N_-_tert_-butanesulfinyl aldimines. _J. Am. Chem. Soc._ 130, 6910–6911 (2008).
Article CAS Google Scholar * Liu, G.C., Cogan, D.A. & Ellman, J.A. Catalytic asymmetric synthesis of _tert_-butanesulfinamide. Application to the asymmetric synthesis of amines. _J.
Am. Chem. Soc._ 119, 9913–9914 (1997). Article CAS Google Scholar * Patterson, A.W. & Ellman, J.A. Asymmetric synthesis of α,α-dibranched propargylamines by acetylide additions to
_N_-_tert_-butanesulfinyl ketimines. _J. Org. Chem._ 71, 7110–7112 (2006). Article CAS Google Scholar * Lo, V.K.-Y., Zhou, C.-Y., Wong, M.-K. & Che, C.-M. Silver(i)-mediated highly
enantioselective synthesis of axially chiral allenes under thermal and microwave-assisted conditions. _Chem. Commun._ 46, 213–215 (2010). Article CAS Google Scholar * Corbett, J.W. et al.
Inhibition of clinically relevant mutant variants of HIV-1 by quinazolinone non-nucleoside reverse transcriptase inhibitors. _J. Med. Chem._ 43, 2019–2030 (2000). Article CAS Google
Scholar * Trost, B.M., Chung, C.K. & Pinkerton, A.B. Stereocontrolled total synthesis of (+)-streptazolin by a palladium-catalyzed reductive diyne cyclization. _Angew. Chem. Intl. Edn._
43, 4327–4329 (2004). Article CAS Google Scholar * Davidson, M.H. & McDonald, F.E. Stereoselective synthesis of D-desosamine and related glycals via tungsten-catalyzed alkynol
cycloisomerization. _Org. Lett._ 6, 1601–1603 (2004). Article CAS Google Scholar * Brennan, C.J., Pattenden, G. & Rescourio, G. Formal synthesis of (+)-lactacystin based on a novel
radical cyclisation of an α-ethynyl substituted serine. _Tetrahedron Lett._ 44, 8757–8760 (2003). Article CAS Google Scholar * Cantel, S. et al. Synthesis and conformational analysis of a
cyclic peptide obtained via i to i+4 intramolecular side-chain to side-chain azide-alkyne 1,3-dipolar cycloaddition. _J. Org. Chem._ 73, 5663–5674 (2008). Article CAS Google Scholar *
Brak, K., Doyle, P.S., McKerrow, J.H. & Ellman, J.A. Identification of a new class of nonpeptidic inhibitors of cruzain. _J. Am. Chem. Soc._ 130, 6404–6410 (2008). Article CAS Google
Scholar * Brak, K. et al. Nonpeptidic tetrafluorophenoxymethyl ketone cruzain inhibitors as promising new leads for Chagas disease chemotherapy. _J. Med. Chem._ 53, 1763–1773 (2010).
Article CAS Google Scholar * Verdoes, M. et al. A nonpeptidic cathepsin S activity-based probe for noninvasive optical imaging of tumor-associated macrophages. _Chem. Biol._ 19, 619–628
(2012). Article CAS Google Scholar * Leyva, M.J. et al. Identification and evaluation of small molecule pan-caspase inhibitors in Huntington's disease models. _Chem. Biol._ 17,
1189–1200 (2010). Article CAS Google Scholar * Deu, E. et al. Functional studies of _Plasmodium falciparum_ dipeptidyl aminopeptidase I using small molecule inhibitors and active site
probes. _Chem. Biol._ 17, 808–819 (2010). Article CAS Google Scholar * Wood, W.J., Patterson, A.W., Tsuruoka, H., Jain, R.K. & Ellman, J.A. Substrate activity screening: a
fragment-based method for the rapid identification of nonpeptidic protease inhibitors. _J. Am. Chem. Soc._ 127, 15521–15527 (2005). Article CAS Google Scholar * Patterson, A.W. et al.
Identification of selective, nonpeptidic nitrile inhibitors of cathepsin S using the substrate activity screening method. _J. Med. Chem._ 49, 6298–6307 (2006). Article CAS Google Scholar
* Inagaki, H. et al. Characterization and optimization of selective, nonpeptidic inhibitors of cathepsin S with an unprecedented binding mode. _J. Med. Chem._ 50, 2693–2699 (2007). Article
CAS Google Scholar * Moss, N. et al. Exploration of cathepsin S inhibitors characterized by a triazole P1-P2 amide replacement. _Bioorg. Med. Chem. Lett._ 22, 7189–7193 (2012). Article
CAS Google Scholar * Moura-Letts, G., Diblasi, C.M., Bauer, R.A. & Tan, D.S. Solid-phase synthesis and chemical space analysis of a 190-membered alkaloid/terpenoid-like library. _Proc.
Natl. Acad. Sci. USA_ 108, 6745–6750 (2011). Article CAS Google Scholar * Cogan, D.A., Liu, G. & Ellman, J. Asymmetric synthesis of chiral amines by highly diastereoselective
1,2-additions of organometallic reagents to _N_-_tert_-butanesulfinyl imines. _Tetrahedron_ 55, 8883–8904 (1999). Article CAS Google Scholar * Shaw, A.W. & deSolms, S.J. Asymmetric
synthesis of α,α-diaryl and α-aryl-α-heteroaryl alkylamines by organometallic additions to _N_-_tert_-butanesulfinyl ketimines. _Tetrahedron Lett._ 42, 7173–7176 (2001). Article CAS Google
Scholar * Chen, B.-L., Wang, B. & Lin, G.-Q. Highly diastereoselective addition of alkynylmagnesium chlorides to N-_tert_-butanesulfinyl aldimines: a practical and general access to
chiral α-branched amines. _J. Org. Chem._ 75, 941–944 (2009). Article Google Scholar * Ding, C.-H., Chen, D.-D., Luo, Z.-B., Dai, L.-X. & Hou, X.-L. Highly diastereoselective synthesis
of _N_-_tert_-butylsulfinylpropargylamines through direct addition of alkynes to _N_-_tert_-butanesulfinimines. _Synlett_ 2006, 1272–1274 (2006). Article Google Scholar Download
references ACKNOWLEDGEMENTS This work was supported by the US National Science Foundation (CHE-1049571). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Chemistry, Yale
University, New Haven, Connecticut, USA Hai-Chao Xu, Somenath Chowdhury & Jonathan A Ellman * Department of Chemistry, Xiamen University, Xiamen, China Hai-Chao Xu Authors * Hai-Chao Xu
View author publications You can also search for this author inPubMed Google Scholar * Somenath Chowdhury View author publications You can also search for this author inPubMed Google Scholar
* Jonathan A Ellman View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS H.-C.X. and S.C. carried out the experiments; J.A.E. designed the
protocol and supervised the project; and H.-C.X. and J.A.E. assembled the manuscript. CORRESPONDING AUTHOR Correspondence to Jonathan A Ellman. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY DATA 1H NMR and 13C NMR spectra of products 16–19 (PDF 515 kb) RIGHTS AND PERMISSIONS Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Xu, HC., Chowdhury, S. & Ellman, J. Asymmetric synthesis of amines using _tert_-butanesulfinamide. _Nat Protoc_ 8, 2271–2280 (2013).
https://doi.org/10.1038/nprot.2013.134 Download citation * Published: 24 October 2013 * Issue Date: November 2013 * DOI: https://doi.org/10.1038/nprot.2013.134 SHARE THIS ARTICLE Anyone you
share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the
Springer Nature SharedIt content-sharing initiative