- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Sickness behavior in humans is characterized by low mood and fatigue, which have been suggested to reflect changes in motivation involving reorganization of priorities. However, it
is unclear which specific processes underlying motivation are altered. We tested whether bacterial endotoxin _E. coli_ lipopolysaccharide (LPS) affected two dissociable constructs of
motivational behavior, ie, effort and reward sensitivity. After familiarization with 5 effort levels, participants made a series of accept/reject decisions on whether the stake offered (1,
4, 8, 12, or 15 apples) was ‘worth the effort’ (10%, 27.5%, 45%, 62.5%, and 80% of maximal voluntary contraction in a hand-held dynamometer). Effort and reward levels were parametrically
modulated to dissociate their influence on choice. Overall, 29 healthy young males were administered LPS (2 ng/kg; _n_=14) or placebo (0.9% saline; _n_=15). The effort-stake task, and
self-reported depression and fatigue were assessed prior to LPS/placebo injection, 2 and 5 h post injection. Cytokines and sickness symptoms were assessed hourly till 8 h after LPS
injection. LPS transiently increased interleukin-6 and tumor necrosis factor-_α_, sickness symptoms, body temperature and self-reported fatigue, and depression post injection relative to
baseline and placebo. These changes were accompanied by LPS-induced decreases in acceptance rates of high-effort options, without significantly affecting reward sensitivity 2 h post
injection, which were partially recovered 5 h post injection. We suggest that LPS-induced changes in motivation may be due to alterations to mesolimbic dopamine. Our behavioral paradigm
could be used to further investigate effects of inflammation on motivational behavior in psychiatric and chronic illnesses. SIMILAR CONTENT BEING VIEWED BY OTHERS EXAGGERATED FRONTOPARIETAL
CONTROL OVER COGNITIVE EFFORT-BASED DECISION-MAKING IN YOUNG WOMEN WITH ANOREXIA NERVOSA Article Open access 28 August 2024 A NEUROMETABOLIC MECHANISM INVOLVING DMPFC/DACC LACTATE IN
PHYSICAL EFFORT-BASED DECISION-MAKING Article Open access 30 August 2024 ACUTE STRESS PROMOTES EFFORT MOBILIZATION FOR SAFETY-RELATED GOALS Article Open access 01 June 2024 INTRODUCTION
Motivational symptoms such as apathy and fatigue are common in patients with psychiatric disorders including depression, schizophrenia, and bipolar disorder (Toomey et al, 1998). A growing
field of research suggests that inflammation may contribute to these motivational symptoms (Felger and Treadway, 2017; Réus et al, 2015; Rosenblat et al, 2014). This is supported by
observations of elevated levels of pro-inflammatory cytokines interleukin-6 (IL-6) and tumor necrosis factor-_α_ (TNF_α_) in people who suffer from chronic major depression (Dowlati et al,
2010; Müller and Schwarz, 2007; Young et al, 2014) and observations of neuroinflammation in patients with bipolar disorder (Rao et al, 2009; Stertz et al, 2013; Yüksel and Öngür, 2010) and
schizophrenia (Garver et al, 2003; Rosenblat et al, 2014). Systemic inflammation in humans typically induces a cluster of non-specific symptoms (ie, sickness behavior) including fatigue,
depression, and apathy (Dantzer, 2001). Studies of sickness behavior in animals suggest that these behavioral changes might be mediated by cytokine effects on the central nervous system
(Dantzer et al, 2014; Felger and Miller, 2012; Swardfager et al, 2016). Following pathogen exposure, pro-inflammatory cytokines are released by activated immune cells to orchestrate the
physiological immunologic response (Dembic, 2015). These pro-inflammatory cytokines also have a critical role in the regulation of immune influences on brain function (Kronfol and Remick,
2000) and have shown to affect dopamine function in mesolimbic brain regions (Neurauter et al, 2008; Capuron et al, 2012). Dopamine has repeatedly been associated with both reward and
effort-based decision making, but it remains to be determined how inflammation affects effort and reward influences on behavior. Several human studies have investigated reward learning and
mesolimbic functioning after treatment with the inflammatory cytokine interferon alpha (INF_α_) or acute inflammation challenges. These studies demonstrated altered reward learning (Harrison
et al, 2015) and reductions in reward-related ventral striatal activity, that was associated with inflammation-induced increases in depression, fatigue, and anhedonia (Capuron et al, 2012;
Dowell et al, 2016; Eisenberger et al, 2010). By contrast, research with animals suggest that inflammation affects effort expenditure, rather than reward processing (Larson et al, 2002;
Larson, 2006; Nunes et al, 2014; Yohn et al, 2016). In a two-choice (high-effort/high-reward _vs_ low-effort/low-reward) paradigm (Salamone et al, 1994), administration of IL-1_β_ shifted
rodent’s choice towards the low-effort/low-reward option. Importantly, reward sensitivity remained intact as high-reward preferences were unaffected (Nunes et al, 2014). Another study
demonstrated that inflammation reduced the overall effort investment (ie, number of responses), whereas the better high-effort/high-reward option was still favored (Vichaya et al, 2014). A
version of this latter paradigm was recently assessed in humans where participants chose between high-effort/high-reward and low-effort/low-reward options. Reward magnitude and probability
was modulated (Lasselin et al, 2016). Although participants selected the high-effort/high-reward options at the same rate during inflammation compared to placebo, they selected a greater
proportion of the high-effort options when the probability to win the reward was high. Thus, participants still performed the high-effort options during inflammation to gain a higher reward,
suggesting that they are still reward sensitive. Paradigms used to date have been limited in the dissociation of reward and effort influences as they typically compare
high-reward/high-effort options with low-reward/low-effort options. Accordingly, in the current investigation, we aimed to test whether systemic inflammation differentially affects reward or
effort processing in healthy human volunteers using a recently developed effort-stake choice paradigm (Bonnelle et al, 2015, 2016). In this paradigm, we parametrically modulate effort and
reward choices by providing options with combinations of different levels of reward and effort, allowing us to dissociate effort and reward influences on choice. Our second aim was to
explore the relationship between changes in motivational behavior and changes in fatigue and depression or pro-inflammatory cytokine response. Informed by current literature highlighting the
role of IL-1_β_, IL-6, and TNF_α_ in various chronic conditions that express motivational symptoms, including in major depressive disorder (Dowlati et al, 2010; Young et al, 2014; (Engler
et al, 2017; Felger and Treadway, 2017), rheumatoid arthritis (Roerink _et al_, 2017a); and cancer-related fatigue (Bower and Lamkin, 2013; Schubert et al, 2007; Smith et al, 2014; Wood and
Weymann, 2013; Roerink et al, 2017a), as well as the effects of acute administration of IL-6 and IL-1_β_ on animal behavior (Nunes et al, 2014; Vichaya et al, 2014; Bonsall _et al_, 2015;
Yohn et al, 2016), we focused our investigation on these three pro-inflammatory cytokines. MATERIALS AND METHODS GENERAL SESSION PROCEDURE This study was part of a larger clinical trial at
the department of intensive care medicine of the Radboudumc in Nijmegen in the Netherlands investigating the effects of human endotoxemia followed by the administration of a live-attenuated
influenza vaccine ‘Fluenz’ on the immune response (ClinicalTrials.gov: NCT02642237). The human endotoxemia sessions took place 1 week before the administration of Fluenz and were therefore
not confounded by this second part of the clinical trial. Participants received either _E. coli_-derived lipopolysaccharide (LPS) at a dose of 2 ng/kg or saline (0.9% NaCl) intravenously.
They were randomly assigned to the LPS or placebo condition on the morning of testing by an unaffiliated research nurse and deblinding of conditions took place after all data had been
collected. To control for individual differences in baseline performance, behavioral testing took place at three time points: session 1: 45 min before injection; session 2: 2 h post
injection and; session 3: 5 h post injection. ‘Timing was based on previous experiences from our group showing that sickness symptoms are limited 2 h after LPS administration, whereas
cytokine levels are still high (Kox et al, 2015; Leentjens et al, 2012).’ All study procedures were in accordance with the declaration of Helsinki, including the latest revisions and
approved by the local medical ethics committee (CMO: 2015/2058). PARTICIPANTS Thirty healthy, non-smoking Caucasian males aged 18–35 years old (median age 21; IQR: 20–23) without any
medical/psychiatric history or current use of (prescription) drugs were recruited by the Radboud University Medical Centre Intensive Care Research Unit (see Table 1; Supplementary Materials
for inclusion and exclusion criteria). All subjects were bachelor or master students from the local universities. To reduce potential variation related to gender or hormonal fluctuations in
female menstrual cycle (Angele et al, 2014, Engler et al, 2016, Karshikoff et al, 2015; Moieni et al, 2015), only male subjects were used. Participants were asked to refrain from food (12 h)
as well as caffeine and alcohol (24 h) before the LPS/placebo challenge. One volunteer was excluded due to vomiting that interfered with task performance during session 2 (LPS group:
_N_=14, placebo group: _N_=15). FORCE-LEVEL FAMILIARIZATION After estimation of maximum voluntary contraction (MVC) (see Supplementary Materials for details on apparatus and MVC estimation),
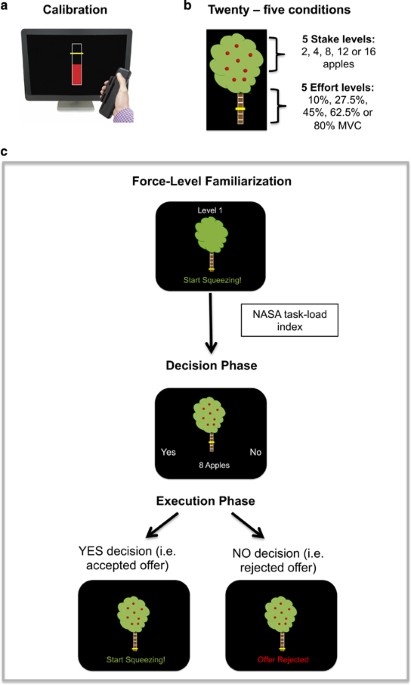
the five effort levels were set as 10%, 27.5%, 45%, 62.5%, and 80% of each individual’s MVC, and represented as sections on the trunk of an apple tree (Figure 1b). Beginning at effort level
1, participants practiced squeezing to the required force and holding their grip at that force for 2 s (Figure 1c). The trunk of the tree filled up with red as the dynamometer was squeezed,
and turned yellow as soon as the required force was reached. Each effort level was performed twice sequentially from level 1 to level 5 using the dominant hand. Force-level familiarizations
were repeated at the start of each session to remind participants of the effort required for each level. EXPERIMENTAL TASK Participants were presented with a series of offers, in the form
of the apple trees, and they had to select YES or NO, by lightly squeezing the right or left dynamometer, depending on if they evaluated the stake offered to be ‘worth the effort’.
Twenty-five possible offers (all combinations of the 5 effort levels and 5 stake levels (1, 4, 8, 12, or 15 apples)) were each sampled four times in a pseudo-random order, totaling 100
trials in each session (Figure 1). To make each judgment behaviorally relevant, participants were told that 26 decisions would be randomly selected for them to perform immediately following
the decision phase. These 26 trials were then presented during an execution phase. If the offer was accepted, participants had 5 s to reach the required effort level and hold it for 2 s. If
they were successful, they received feedback stating how many apples they had won. If the offer was rejected, the tree appeared on screen with the message ‘offer rejected’, meaning they were
not able to attempt to win the apples offered (Figure 1c). Participants were told at the start of the day they would be rewarded based on the number of apples (worth 3 cents each) they
gathered during this execution phase. Ten trials were practiced before session 1 to familiarize them with the stake/effort relationships. To control for changes in perceived task demand,
participants performed a NASA task-load index questionnaire (Hart and Staveland, 1988) after each familiarization session. Participants rated temporal, physical, and mental demand;
frustration, effort required and their performance for each effort level. MEASUREMENT OF SICKNESS BEHAVIOR AND MOOD Physical sickness symptoms were measured before LPS administration (T=0)
and at 30-min intervals until 8 h after LPS administration (17 measures). Participants were asked to rate from 0 (absent) to 5 (very severe) the severity of six common symptoms: nausea,
headache, muscle aches, back pain, shivers, and vomiting. Self-reported mood was assessed using the depression and fatigue subscales of the profile of moods state questionnaire (POMS (McNair
et al, 1971)) at the start of each session (see Supplementary Materials for details on the subscale items). MEASUREMENT OF CYTOKINES IN PLASMA EDTA-anticoagulated blood was collected at: 1,
1.5, 2, 3, 4, 6, and 8 h after LPS administration, centrifuged (2000 _g_, 4 °C, 10 min), and stored at −80 °C until analysis. Plasma concentrations of cytokines of interest (TNF_α_ and
IL-6) were measured using a simultaneous (entered together in one batch) Luminex assay (R&D systems; Abingdon Science Park, UK, Human high sensitivity cytokine kit, catalog numbers
LHSCM000, LHSCM210, LHSCM206, www.rndsystems.com). Statistical analyses to calculate plasma concentrations were performed using Graphpad Prism version 5.0 (Graphpad Software, San Diego, CA,
USA). Lower detection limits in plasma and intra-assay coefficients of variation (C.V.) were; 0.22 pg/ml (C.V. 1.54%) for TNF_α_ and <0.86 pg/ml (C.V. 0.92%) for IL-6. We initially also
aimed at assessing IL1-Ra and IL1-b because of the suggested association between IL1 and fatigue (Roerink et al, 2017a). Unfortunately, luminex assays of IL1-Ra failed and measures were
considered unreliable as concentrations of IL1-Ra exceeded the upper detection limit (>15 296 pg/ml, C.V.=0.046%). IL-1_β_ was excluded from analyses because plasma concentrations did not
exceed lower detection limits at 2 h post injection (0.79 pg/ml, C.V.=0.30%). STATISTICAL ANALYSIS BEHAVIORAL TASK The percentage of accepted offers for each of the 25 conditions (5 effort
and 5 stake levels) was the key variable for each participant. We first tested whether LPS induced a change in choice behavior between session 1 and session 2. Acceptance rates were entered
into a 5 (effort level) × 5 (stake level) × 2 (time) × 2 (groups) AVOVA. On the basis of our hypothesis, we were specifically interested in whether LPS affected effort and/or stake
sensitivity. Accordingly, our tests of interests were the effort × time × stake × group interaction and, if significant, the effort × time × group and stake × time × group interactions. If
LPS induced a significant change in choice behavior, we subsequently ran an ANOVA comparing session 1 and session 3 to test whether the LPS-induced changes in choice behavior recovered to
baseline. These analyses were repeated with generalized estimating equation (GEE) using a binary logistic model and exchangeable working correlation structure, which is better suited for
binomially distributed categorical outcomes. Effort level, stake level, and trial (1–100) were mean centered and entered as continuous variables, session and group (LPS/placebo) were entered
as factors, and the model contained all main effects and interactions. SUBJECTIVE MEASURES, CYTOKINES, AND PHYSIOLOGY A total score for sickness symptoms was calculated for each subject at
each time point. Febrile response and sickness symptoms were entered into a repeated-measures ANOVA with the factors time (17 levels) and group. Plasma concentrations of cytokines (TNF_α_
and IL-6) were entered into a repeated-measures ANOVA with the factors time (8 levels) and group. Total scores for depression and fatigue were calculated as mean score on the POMS subscales
for each session. A repeated-measures ANOVA with the factors time (3 levels) and group was performed for fatigue and depression scores separately. Similar to our behavioral analysis, we
first assessed LPS-induced changes between session 1 and 2. If significant, we assessed whether changes recovered to baseline by comparing session 1 and 3. _Post-hoc_ Bonferroni-corrected
independent _t_-tests were calculated, where appropriate. RELATIONSHIP BETWEEN EFFORT/STAKE SENSITIVITY AND MOOD AND CYTOKINES To assess whether LPS-induced changes in behavior were
associated with changes in mood and/or cytokines, we first calculated the individual levels of effort and reward sensitivity via a binomial logistic regression in Matlab with effort and
stake level as predictors and the decisions (yes/no) as dependent variable for each subject. The _β_’s were standardized by dividing by the standard error to minimize the impact of inflated
_β_’s (Apps et al, 2015) (Supplementary Table S2). Change (session 2−session 1) in the standardized _β_’s were used to assess relationships with LPS-induced changes in mood and/or cytokines.
Two stepwise multiple regressions were used with change in effort sensitivity and change in stake sensitivity as dependent variable. Change in depression, change in fatigue, peak
concentration of IL-6, and peak concentration of TNF_α_ were entered as predictor variables in both regressions. All regressions were computed using only data from the LPS group. Statistical
threshold for all stepwise multiple regression analyses were Bonferroni-corrected for multiple comparisons. CONTROL ANALYSES To assess whether LPS effects were explained by alterations in
perceived physical demand, we assessed LPS effects on total scores of the NASA task-load index questionnaire (sum of each section) using a repeated-measures ANOVA with the factors effort,
time, and group. We additionally assessed LPS effects on the NASA task-load index subscales ‘physical demand’ (How physically demanding was the task?) and ‘effort’ (how hard did you have to
work to accomplish the task?) using repeated-measures ANOVA with the factors effort, time and group, and on the sickness symptom ‘muscle aches’ using repeated-measures ANOVA with the factors
time and group. When no groups’ differences were found, we tested whether LPS effects on behavior remained significant when the NASA subscales or the sickness symptom ‘muscle aches’ were
added as covariate into the ANOVA on acceptance rates. Finally, we assessed the relationship between standardized _β_’s from the binomial logistic regression and other variables that may
confound the behavioral results (ie, total sickness symptoms, muscle aches, febrile response, NASA physical demand, and NASA effort) within the LPS group using Pearson’s correlation
analysis. Total sickness symptoms and febrile response could not be added as covariate in the ANOVA because they were significantly affected by LPS (see ‘Results’) and therefore violate the
assumption of homogeneity of regression slopes (Miller and Chapman, 2001). RESULTS BEHAVIORAL TASK Results are presented in Figure 2 and Supplementary Tables S1 and S2. As shown previously
in healthy people (Bonnelle et al, 2015, 2016), there were significant main effects of effort and stake on acceptance rates, demonstrating that both groups were sensitive to the effort and
stake manipulations (acceptance rates increase with higher stake and lower effort levels) (stake: F(4,108)=131.2, _p_<0.001, effort: F(4,108)=117.2, _p_<0.001). LPS effects on stake
differed significantly from LPS effects on effort (stake × effort × time × group: (F(16,432)=1.81, _p_=0.028), with significant group effects on effort-related acceptance rates (effort ×
time × group: F(4,108)=3.2, _p_=0.016), but not stake-related acceptance rates (stake × time × group: F(4,108)=0.4, _p_>0.7). Breakdown of the interaction by group revealed that
effort-related acceptance rates were reduced in the LPS group (stake × effort × time: F(16,208)=3.12, _p_<0.001; effort × time: F(4,56)=4.93, _p_=0.002; stake × time: F(4,56)=1.77,
_p_=0.15), but not in the placebo group (stake × effort × time: F(16,208)=1.17, _p_=0.29; effort × time: F(4,56)=1.13, _p_=0.34; stake × time: F(4,56)=2.28, _p_=0.11). Further breakdown of
the effort × time × group interaction by session revealed that there was no between group difference at session 1 on any of the effort levels (all _p_>0.05), and that the LPS group
accepted less offers than the placebo group for the highest effort level during session 2 (80%: _T_(27)=−2.695, _p_=0.012; 62.5%: _T_(27)=−1.843, _p_=0.076). This between group difference on
effort was not present during session 3, compatible with partial recovery (effort × stake × time × group: F(16,432)=1.295, _p_=0.196; effort × time × group (F(4,108)=0.366, _p_=0.833; stake
× time × group (F(4,108)=0.999, _p_=0.411). These findings were confirmed with GEE (stake × effort × time × group: _β_=−0.026, SD=0.13, _p_=0.043; effort × time × group: _β_=−0.85, SD=0.22,
_p_<0.001; stake × time × group: _β_=0.36, SD=0.19, _p_=0.062). Breakdown of the interaction by group revealed that effort but not stake-related acceptance rates were reduced in the LPS
group (stake × effort × time: _β_=0.24, SD=0.11, _p_=0.021; effort × time: _β_=0.75, SD=0.20, _p_<0.001; stake × time: _β_=−0.12, SD=0.13, _p_=0.35), whereas the trend observed for the
stake × time × group interaction was driven by a trend in the placebo group (stake × effort × time: _β_=−0.02, SD=0.08, _p_=0.81; effort × time: _β_=−0.14, SD=0.10, _p_=0.17; stake × time:
_β_=0.27, SD=0.14, _p_=0.053). Further breakdown of the effort × time × group interaction by session revealed that there was a between group difference at session 2 (stake × effort × group:
_β_=−0.31, SD=0.13, _p_<0.016; effort × group: _β_=1.03, SD=0.25, _p_<0.001; stake × group: _β_=−0.46, SD=0.24, _p_=0.053) but not at session 1 (stake × effort × group: _β_=−0.066,
SD=0.23, _p_=0.42) or session 3 (stake × effort × group: _β_=−0.184, SD=0.12, _p_=0.14) (Supplementary Tables S3 and S4). Total rewards obtained in the execution phase ranged between
€2.67–€6.42 for session 1, €1.25–€6.39 for session 2, and €1.41–€6.39 for session 3 and did not differ between groups (all _p_>0.05) (Supplementary Table S2). All subjects were able to
successfully perform all effort levels twice before each session, indicating that LPS did not affect the ability to perform high-effort trials. SUBJECTIVE MEASURES, CYTOKINES, AND PHYSIOLOGY
LPS, but not placebo, induced an increase in sickness symptoms (group × time: F(1,26)=18.9, _p_<0.001), which peaked at 1.5 h post injection. Importantly, sickness symptoms were
significantly higher in the LPS group relative to the placebo group at session 2, whereas no group differences were observed at session 1 prior to injection, nor at session 3 at 5 h post
injection (session 1 _T_(27)=1.37, _p_>0.1; session 2: _T_(27)=2.15, _p_<0.05; session 3: _T_(27)=0.30, _p_>0.7, Figure 3a; Supplementary Table S5). LPS resulted in a 1±0.6 °C
(mean±SD) increase in temperature (F(16,432)=13.4, _p_<0.001) and marked increases in all cytokines of interest at session 2 (IL-6: F(7,189)=32.48, _p_<0.001; TNF_α_: F(7,189)=88.83,
_p_<0.001) (Figure 3b–d). Temperature and cytokines reduced back to baseline by 8 h from injection. The placebo group showed no change in any of the cytokines throughout the whole
recorded period. LPS affected self-reported depression and fatigue levels (depression time × group: F(1,27)=10.997, _p_=0.003; fatigue time × group: F(1,27)=23.6, _p_<0.001).
Specifically, the LPS group reported feeling significantly more depressed and fatigued than the placebo group during session 2 (depression: _T_(27)=3.609, _p_<0.001; fatigue:
_T_(27)=4.806, _p_<0.001), but not session 1 (depression: _T_(27)=1.517, _p_=0.141; fatigue: _T_(27)=0.915, _p_=0.368). The time × group interaction effect was no longer significant for
fatigue or depression when looking at session 3 _vs_ session 1 (depression: _T_(27)=1.579, _p_=220; fatigue: _T_(27)=2.477, _p_<0.127). However, direct comparisons revealed that the LPS
group remained significantly more depressed than the placebo group during session 3, but only marginally more fatigued (depression: _T_(27)=2.221, _p_=0.035; fatigue: _T_(27)=1.942,
_p_=0.063) suggesting only partial recovery for fatigue (Figure 4a). RELATIONSHIP BETWEEN EFFORT/STAKE SENSITIVITY AND MOOD AND CYTOKINES No relationship between changes in motivational
behavior and mood or cytokines were observed: POMS scores for depression and fatigue, IL-6 or TNF did not remain in the stepwise multiple regression analyses as significant predictors for
LPS-induced change in effort or stake sensitivity (all _p_>0.1, Supplementary Table S6). CONTROL ANALYSES The LPS group did not differ from the placebo group on the total NASA score or
any of the NASA subscales (no significant interaction effects, minimum _p_>0.05, Figure 4b; Supplementary Table S5) indicating that LPS did not affect perceived (physical) demand of the
requested effort levels. LPS effects on muscle aches did not differ between the groups (F(1,27)=1.11, _p_=0.30). Adding the factor ‘physical demand’ as covariates to the main analyses did
not affect our main result (F(16,400)=1.802, _p_=0.029), and no relationships were observed between ‘physical demand’ and effort sensitivity (_r_(14)=0.34, _p_=0.02). However, adding the
subscale ‘effort’ as covariate to the main analyses resulted in a trend for the effort × stake × time interaction (F(16,400)=1.65, _p_=0.054). Change in NASA effort was also marginally
correlated with change in effort sensitivity (_r_=0.369, _p_=0.053) (Supplementary Table S6). Adding muscle aches a covariate in the analysis of decisions did not affect our main result
(F(16,400)=1.70, _p_=0.044, Supplementary Table S1). No relationships between LPS effects on behavior and muscle aches, total sickness symptoms, and febrile response were observed (all
_p_>0.05, Supplementary Table S6). DISCUSSION In this study, we used a relatively new paradigm that parametrically modulates offers with respect to stake and the effort required to obtain
that reward (Bonnelle et al, 2015, 2016). We demonstrate that experimentally induced endotoxemia using LPS in humans reduces otherwise healthy participant’s willingness to accept
high-effort options, without significantly altering reward sensitivity. It has been suggested that sickness behavior is an adaptive motivational state that involves reprioritization of the
costs and benefits of expending effort, rather than simply being general physical weakness (Dantzer, 2001). Our results support this claim: LPS reduced acceptance rates of high-effort
options, whereas the ability to perform the task was not changed. All participants were able to successfully perform the effort levels during a familiarization phase prior to their decisions
and participants reported no differences in perceived demand to perform the task as indexed by the NASA task-load index. This suggests that the changes we observed are due to altered
motivation, rather than a change in physical strength or ability. Changes in motivation are common across a broad range of psychiatric and medical conditions (Fervaha et al, 2015; Kostić and
Filippi, 2011; Sinha et al, 2013; Starkstein and Pahissa, 2014). There is increasing evidence that inflammation may have an important role in development of amotivated states (Leboyer et
al, 2012; Miller et al, 2009; Potvin et al, 2008; Réus et al, 2015; Bonaccorso et al, 2001; Capuron et al, 2012; Majer et al, 2008; Wichers et al, 2005). Here, we found that LPS increased
cytokines and concomitantly altered motivational behavior and mood supporting the concept that inflammation could have a role in development of symptoms such as depression and fatigue
(Dowell et al, 2016; Engler et al, 2017; Felger and Treadway, 2017; Karshikoff et al, 2017; Miller and Raison, 2016). However, we could not demonstrate direct relationships between
LPS-induced behavioral changes and cytokine concentrations or mood. This could be due to our small sample size, lacking the power to detect such associations. In addition, the immune
manipulation we used (LPS) induced robust increases with little variation in multiple cytokines that strongly interact with one another, making it difficult to disentangle which cytokine is
responsible for the observed change in behavior. Future studies that induce more variation, eg, by using different dosages or selectively stimulate or inhibit cytokines could provide more
insight on the role of specific cytokines on change in behavior. Although many studies have suggested relationships between motivational behavior and mood, actual reports of these
relationships have been limited (Lasselin et al, 2016; Salamone et al, 2016). One reason for this could be that the POMS was not sensitive enough to detect subtle alterations in depressive
mood and fatigue. Alternatively, because our task dissociated between the sub-constructs of motivation (effort and reward) (Berridge _et al_, 2009), it may have captured behavior that is not
necessarily reflected by the subjective reports of fatigue and depression (Karshikoff et al, 2017). To the best of our knowledge, there has only been one previous human study that
investigated effort and reward processing during inflammation (Lasselin et al, 2016). That pioneering investigation used two-option choices (high-reward/high-effort _vs_
low-reward/low-effort) and showed that after LPS the high-effort option was still favored, more so when the probability of gaining the reward was also high. There was no difference between
LPS and placebo conditions in the number of high-effort choices. Although our results may at first appear to contradict these findings, both studies demonstrated that reward sensitivity was
not affected by LPS. In addition, the two studies differed in important ways. First, in Lasselin _et al_’s forced-choice design, participants had to perform an action on each trial (meaning
they updated their experience of the effort options), and they chose to perform the high-effort actions to gain a higher reward at the same rate as after placebo. In our design, participants
only had the option to perform actions they considered ‘worth the effort’ for the reward offered, ie, they could choose to do nothing, which led to a reduction in choice of high-effort
options during inflammation. Furthermore, our paradigm parametrically modulated effort and reward, rather than offering two-option choices. In this way, our task allowed participants to gain
the high rewards at a lower effort level, meaning gaining the largest reward was not contingent on putting in the highest effort. Second, the previous human study (Lasselin et al, 2016) had
a probability dimension, the likelihood of gaining the reward on high-effort trial was variable, an aspect which we did not test. Our task was simpler: reward was always given if the
participant reached the effort level required. Finally, the previous investigation tested their participants 4 h post LPS, while we tested participants 2 h post LPS, which might be why our
subjects were more effort sensitive. Indeed, the alterations in effort sensitivity were partly recovered 5 h after LPS. One potential mechanisms through which cytokines can affect
motivational behavior is through interference with brain dopamine function (Felger and Treadway, 2017). Indeed, effort- and reward-related components of motivated behavior have consistently
been linked to dopamine signaling (Chong et al, 2015; Skvortsova et al, 2017; Wardle et al, 2011). For example, using a variation of the task reported here, Chong _et al_ 2015 observed that
patients with Parkinson’s disease, who often experience motivational symptoms, were willing to put in more effort to gain rewards when they were ON dopaminergic drugs compared to when they
were OFF them. Animal work has also shown that dopamine depletion in the nucleus accumbens causes similar changes in effort-based choice behavior as observed here (Randall et al, 2012).
However, previous neuroimaging work has mainly focused on LPS effects on reward-related processes, showing reduced reward-related signals in the ventral striatum (Capuron et al, 2012;
Eisenberger et al, 2010; Harrison et al, 2015). Given the partial dissociable brain networks of effort and reward processing (Klein-Flügge et al, 2016; Skvortsova et al, 2014), the neural
alterations related to LPS effects on effort-based choice remained to be determined. We unfortunately do not have brain imaging data to compare to previous work (Capuron et al, 2012;
Eisenberger et al, 2010; Harrison et al, 2015). Future studies are needed to better dissociate the neural mechanisms of inflammation effects on effort- and reward-related processes. Insights
into immune-to-brain pathways gained from acute immune manipulation studies are important for better mechanistic understanding of motivational deficits in psychiatric conditions, and could
lead to new pharmaceutical targets. Indeed, dopamine enhancing drugs like methylphenidate or levodopa can reverse inflammation effects (Yohn, 2016; Bonsall, 2015; Felger et al, 2015) and has
some effects on reducing fatigue in humans (Blockmans et al, 2006; Kerr et al, 2012; Mendonça et al, 2007; Minton et al, 2011). It remains to be investigated whether other dopamine
manipulations would also reverse inflammation effects on motivational symptoms. Here we show that our task is sensitive to immune manipulation on dissociable sub-constructs of motivational
behavior (reward and effort) in humans. This paradigm is therefore promising for further human research on immune-mediated changes in motivational behavior, and for testing of
pharmacological targets to treat motivational symptoms. These could include targets at the immunological level by inhibiting pro-inflammatory cytokines; a method that has reduced fatigue
symptoms in some medical conditions (Omdal and Gunnarsson, 2005; Roerink _et al_, 2017b), but also targets at the central level, affecting dopamine function, for patients groups in which
immune-alterations are less prominent (Roerink et al, 2017a). This study has several limitations. First, the sample size was small, lacking power to detect relevant associations between
individual immune responses, motivated behavior, and mood. Second, we used a relatively high dose of LPS (2 ng/kg). This inherently also induce sickness symptoms that could affect the
blindness of the conditions and potentially confound task performance. Although we show that our effects are specific to effort and not reward-based decisions, and that sickness symptoms
were not directly correlated with effort-based choice within the LPS group, it is unfortunately statistically impossible to dissociate sickness symptoms from behavioral alterations, as these
factors are not independent (Miller and Chapman, 2001). Simply lowing the dose might not help as this inherently also reduces the behavioral effects, while still inducing significant
increases in sickness symptoms (Benson et al, 2017). Instead, future studies might potentially be able to dissociate these factors, eg, by assessing effects of co-administration with
centrally acting drugs that do not affect sickness symptoms. Finally, we tested only men, whereas clinical work indicates that motivational symptoms such as depression are more prone in
woman (Altemus et al, 2014). In addition, several studies highlight the importance of sex differences in immune–brain interactions that likely mediate the LPS effects on mood and behavior
(Engler et al, 2016; Karshikoff et al, 2015; Moieni et al, 2015). This, therefore, limits generalizability of our results. In summary, experimental endotoxemia reduced, otherwise, healthy
participant’s willingness to engage in high-effort options, while reward sensitivity was not significantly altered. This change in motivation was not due to the task being perceived as more
effortful. Endotoxemia concomitantly induced an increase in subjective reports of depression and fatigue. The behavioral paradigm used in this study provides a human model to further
investigate brain mechanisms underlying inflammation effects on motivational behavior. A better understanding of these mechanisms in humans will be important for further development and
testing of pharmaceutical targets to treat motivational deficits in psychiatric disorders. FUNDING AND DISCLOSURE This research was funded by a Wellcome Trust Principal Fellowship awarded to
Prof Masud Husain and supported by the Oxford NIHR BRC and CRF. This work was also supported by an EFRO Grant (2011-013287). The authors declare no conflict of interest. REFERENCES *
Altemus M, Sarvaiya N, Neill Epperson C (2014). Sex differences in anxiety and depression clinical perspectives. _Front Neuroendocrinol_ 35: 320–330. Article PubMed PubMed Central Google
Scholar * Angele MK, Pratschke S, Hubbard WJ, Chaudry IH (2014). Gender differences in sepsis. _Virulence_ 5: 12–19. Article PubMed Google Scholar * Apps MAJ, Grima LL, Manohar S, Husain
M (2015). The role of cognitive effort in subjective reward devaluation and risky decision-making. _Sci Rep_ 5: 1–11. Article Google Scholar * Benson S, Engler H, Wegner A, Rebernik L,
Spreitzer I, Schedlowski M _et al_ (2017). What makes you feel sick after inflammation? Predictors of acute and persisting physical sickness symptoms induced by experimental endotoxemia.
_Clin Pharmacol Therap_ 102: 141–151. Article CAS Google Scholar * Berridge KC, Robinson TE, Aldridge JW (2009). Dissecting components of reward: “liking”, “wanting”, and learning. _Curr
Opin Pharmacol_ 9: 65–73. Article CAS PubMed PubMed Central Google Scholar * Blockmans D, Persoons P, Van Houdenhove B, Bobbaers H (2006). Does methylphenidate reduce the symptoms of
chronic fatigue syndrome? _Am J Med_ 119: e23–e30. Article Google Scholar * Bonaccorso S, Puzella A, Marino V, Pasquini M, Biondi M, Artini M _et al_ (2001). Immunotherapy with
interferon-alpha in patients affected by chronic hepatitis C induces an intercorrelated stimulation of the cytokine network and an increase in depressive and anxiety symptoms. _Psychiatry
Res_ 105: 45–55. Article CAS PubMed Google Scholar * Bonnelle V, Manohar S, Behrens T, Husain M (2016). Individual differences in premotor brain systems underlie behavioral apathy.
_Cereb Cortex_ 26: 807–819. PubMed Google Scholar * Bonnelle V, Veromann KR, Burnett Heyes S, Lo Sterzo E, Manohar S, Husain M (2015). Characterization of reward and effort mechanisms in
apathy. _J Physiol_ 109: 16–26. Google Scholar * Bonsall DR, Kim H, Tocci C, Ndiaye A, Petronzio A, McKay-Corkum G _et al_ (2015). Suppression of locomotor activity in female C57Bl/6J mice
treated with interleukin-1_β_: investigating a method for the study of fatigue in laboratory animals. _PLoS ONE_ 10: e0140678. Article PubMed PubMed Central Google Scholar * Bower JE,
Lamkin DM (2013). Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. _Brain Behav Immun_ 30: S48–S57. Article CAS PubMed Google Scholar
* Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ _et al_ (2012). Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa
administration. _Arch Gen Psychiatry_ 69: 1044–1053. Article CAS PubMed PubMed Central Google Scholar * Chong TT-J, Bonnelle V, Manohar S, Veromann K-R, Muhammed K, Tofaris GK _et al_
(2015). Dopamine enhances willingness to exert effort for reward in Parkinson’s disease. _Cortex_ 69: 40–46. Article PubMed PubMed Central Google Scholar * Dantzer R (2001).
Cytokine-induced sickness behavior: where do we stand? _Brain Behav Immun_ 15: 7–24. Article CAS PubMed Google Scholar * Dantzer R, Heijnen CJ, Kavelaars A, Laye S, Capuron L (2014). The
neuroimmune basis of fatigue. _Trends Neurosci_ 37: 39–46. Article CAS PubMed Google Scholar * Dembic Z _The Cytokines of the Immune System: The Role of Cytokines in Disease Related to
Immune Response_. Academic Press: Amsterdam; (2015). Google Scholar * Dowell NG, Cooper EA, Tibble J, Voon V, Critchley HD, Cercignani M _et al_ (2016). Acute changes in striatal
microstructure predict the development of interferon-alpha induced fatigue. _Biol Psychiatry_ 79: 320–328. Article CAS PubMed PubMed Central Google Scholar * Dowlati Y, Herrmann N,
Swardfager W, Liu H, Sham L, Reim EK _et al_ (2010). A meta-analysis of cytokines in major depression. _Biol Psychiatry_ 67: 446–457. Article CAS PubMed Google Scholar * Eisenberger NI,
Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR (2010). Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. _Biol Psychiatry_ 68: 748–754. Article
CAS PubMed PubMed Central Google Scholar * Engler H, Benson S, Wegner A, Spreitzer I, Schedlowski M, Elsenbruch S (2016). Men and women differ in inflammatory and neuroendocrine
responses to endotoxin but not in the severity of sickness symptoms. _Brain Behav Immun_ 52: 18–26. Article CAS PubMed Google Scholar * Engler H, Brendt P, Wischermann J, Wegner A,
Röhling R, Schoemberg T _et al_ (2017). Selective increase of cerebrospinal fluid IL-6 during experimental systemic inflammation in humans: association with depressive symptoms. _Mol
Psychiatry_ 22: 1448–1454. Article CAS PubMed Google Scholar * Felger JC, Hernandez CR, Miller AH (2015). Levodopa reverses cytokine-induced reductions in striatal dopamine release. _Int
J Neuropsychopharmacol_ 18. doi: 10.1093/ijnp/pyu084. * Felger JC, Miller AH (2012). Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory
malaise. _Front Neuroendocr_ 33: 315–327. Article CAS Google Scholar * Felger JC, Treadway MT (2017). Inflammation effects on motivation and motor activity: role of dopamine.
_Neuropsychopharmacology_ 42: 216–241. Article CAS PubMed Google Scholar * Fervaha G, Duncan M, Foussias G, Agid O, Faulkner GE, Remington G (2015). Effort-based decision making as an
objective paradigm for the assessment of motivational deficits in schizophrenia. _Schizophr Res_ 168: 483–490. Article PubMed Google Scholar * Garver DL, Tamas RL, Holcomb JA (2003).
Elevated interleukin-6 in the cerebrospinal fluid of a previously delineated schizophrenia subtype. _Neuropsychopharmacology_ 28: 1515–1520. Article CAS PubMed Google Scholar * Harrison
NA, Voon V, Cercignani M, Cooper EA, Pessiglione M, Critchley HD (2015). Archival report a neurocomputational account of how inflammation enhances sensitivity to punishments versus rewards.
_Biol Psychiatry_ 80: 1–9. Google Scholar * Hart SG, Staveland LE (1988). Development of NASA-TLX (Task Load Index): results of empirical and theoretical research. _Adv Psychol_ 52:
139–183. Article Google Scholar * Kerr CW, Drake J, Milch RA, Brazeau DA, Skretny JA, Brazeau GA _et al_ (2012). Effects of methylphenidate on fatigue and depression: a randomized,
double-blind, placebo-controlled trial. _J Pain Symptom Manage_ 43: 68–77. Article CAS PubMed Google Scholar * Kostić VS, Filippi M (2011). Neuroanatomical correlates of depression and
apathy in Parkinson’s disease: magnetic resonance imaging studies. _J Neurol Sci_ 310: 61–63. Article PubMed Google Scholar * Karshikoff B, Lekander M, Soop A, Lindstedt F, Ingvar M,
Kosek E _et al_ (2015). Modality and sex differences in pain sensitivity during human endotoxemia. _Brain Behav Immun_ 46: 35–43. Article CAS PubMed Google Scholar * Karshikoff B,
Sundelin T, Lasselin J (2017). Role of inflammation in human fatigue: relevance of multidimensional assessments and potential neuronal mechanisms. _Front Immunol_ 8: 21. Article PubMed
PubMed Central Google Scholar * Klein-Flügge MC, Kennerley SW, Friston K, Bestmann S (2016). Neural signatures of value comparison in human cingulate cortex during decisions requiring an
effort-reward trade-off. _J Neurosci_ 36: 10002–10015. Article PubMed PubMed Central Google Scholar * Kronfol Z, Remick DG (2000). Cytokines and the brain: implications for clinical
psychiatry. _Am J Psychiatry_ 157: 683–694. Article CAS PubMed Google Scholar * Kox M, van Eijk LT, Verhaak T, Frenzel T, Kiers HD, Gerretsen J _et al_ (2015). Transvenous vagus nerve
stimulation does not modulate the innate immune response during experimental human endotoxemia: a randomized controlled study. _Arthritis Res Therapy_ 17: 1–9. Article Google Scholar *
Larson SJ (2006). Lipopolysaccharide and interleukin-1_β_ decrease sucrose intake but do not affect expression of place preference in rats. _Pharmacol Biochem Behav_ 84: 429–435. Article
CAS PubMed Google Scholar * Larson SJ, Romanoff RL, Dunn AJ, Glowa JR (2002). Effects of interleukin-1_β_ on food-maintained behavior in the mouse. _Brain Behav Immun_ 16: 398–410.
Article CAS PubMed Google Scholar * Lasselin J, Treadway MT, Lacourt TE, Soop A, Olsson MJ, Karshikoff B _et al_ (2016). Lipopolysaccharide alters motivated behavior in a monetary reward
task: a randomized trial. _Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol_ 42: 801–810. Article Google Scholar * Leboyer M, Soreca I, Scott J, Frye M, Henry C, Tamouza R _et
al_ (2012). Can bipolar disorder be viewed as a multi-system inflammatory disease? _J Affect Disord_ 141: 1–10. Article PubMed PubMed Central Google Scholar * Leentjens J, Kox M, Koch
RM, Preijers F, Joosten LAB, van der Hoeven JG _et al_ (2012). Reversal of immunoparalysis in humans _in vivo_. _Am J Resp Crit Care Med_ 186: 838–845. Article CAS PubMed Google Scholar
* Majer M, Welberg LAM, Capuron L, Pagnoni G, Raison CL, Miller AH (2008). IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic
hepatitis C. _Brain Behav Immun_ 22: 870–880. Article CAS PubMed PubMed Central Google Scholar * McNair DM, Lorr M, Droppleman LF (1971) _The Profile of Mood States._ Educational and
Industrial Testing Service: San Diego, CA, USA. * Mendonça DA, Menezes K, Jog MS (2007). Methylphenidate improves fatigue scores in Parkinson disease: a randomized controlled trial. _Mov
Disord Off J Mov Disord Soc_ 22: 2070–2076. Article Google Scholar * Miller AH, Maletic V, Raison CL (2009). Inflammation and its discontents: the role of cytokines in the pathophysiology
of major depression. _Biol Psychiatry_ 65: 732–741. Article CAS PubMed PubMed Central Google Scholar * Miller AH, Raison CL (2016). The role of inflammation in depression: from
evolutionary imperative to modern treatment target. _Nat Rev Immunol_ 16: 22–34. Article CAS PubMed PubMed Central Google Scholar * Miller GA, Chapman JP (2001). Misunderstanding
analysis of covariance. _J Abnorm Psychol_ 110: 40–48. Article CAS PubMed Google Scholar * Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI (2015). Sex differences in
depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. _Neuropsychopharmacology_ 40: 1709–1716. Article CAS PubMed PubMed
Central Google Scholar * Minton O, Richardson A, Sharpe M, Hotopf M, Stone PC (2011). Psychostimulants for the management of cancer-related fatigue: a systematic review and meta-analysis.
_J Pain Symptom Manage_ 41: 761–767. Article CAS PubMed Google Scholar * Müller N, Schwarz M (2007). The immune-mediated alteration of serotonin and glutamate: towards an integrated view
of depression. _Mol Psychiatry_ 12: 988–1000. Article PubMed Google Scholar * Neurauter G, Schrocksnadel K, Scholl-Burgi S, Sperner-Unterweger B, Schubert C, Ledochowski M _et al_
(2008). Chronic immune stimulation correlates with reduced phenylalanine turnover. _Curr Drug Metab_ 9: 622–627. Article CAS PubMed Google Scholar * Nunes EJ, Randall PA, Estrada A,
Epling B, Hart EE, Lee CA _et al_ (2014). Effort-related motivational effects of the pro-inflammatory cytokine interleukin 1-beta: Studies with the concurrent fixed ratio 5/chow feeding
choice task. _Psychopharmacology_ 231: 727–736. Article CAS PubMed Google Scholar * Omdal R, Gunnarsson R (2005). The effect of interleukin-1 blockade on fatigue in rheumatoid
arthritis—a pilot study. _Rheumatol Int_ 25: 481–484. Article CAS PubMed Google Scholar * Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E (2008). Inflammatory cytokine
alterations in schizophrenia: a systematic quantitative review. _Biol Psychiatry_ 63: 801–808. Article CAS PubMed Google Scholar * Randall PA, Pardo M, Nunes EJ, López Cruz L, Vemuri VK,
Makriyannis A _et al_ (2012). Dopaminergic modulation of effort-related choice behavior as assessed by a progressive ratio chow feeding choice task: pharmacological studies and the role of
individual differences. _PLoS ONE_ 7: e47934. Article CAS PubMed PubMed Central Google Scholar * Rao J, Harry G, Rapoport S, Kim H (2009). Increased excitotoxicity and neuroinflammatory
markers in postmortem frontal cortex from bipolar disorder patients. _Mol Psychiatry_ 15: 384–392. Article PubMed PubMed Central Google Scholar * Réus GZ, Fries GR, Stertz L, Badawy M,
Passos IC, Barichello T _et al_ (2015). The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. _Neuroscience_ 300: 141–154. Article PubMed
Google Scholar * Roerink ME, van der Schaaf ME, Dinarello CA, Knoop H, van der Meer JW (2017) a). Interleukin-1 as a mediator of fatigue in disease: a narrative review. _J Neuroinflamm_ 14:
16. Article Google Scholar * Roerink ME, Bredie SJHJH, Heijnen M, Dinarello CA, Knoop H, van der Meer JW (2017b). Cytokine inhibition in patients with chronic fatigue syndrome: a
randomized trial. _Ann Intern Med_ 166: 557–564. Article PubMed Google Scholar * Rosenblat JD, Cha DS, Mansur RB, McIntyre RS (2014). Inflamed moods: a review of the interactions between
inflammation and mood disorders. _Prog Neuropsychopharmacol Biol Psychiatry_ 53: 23–34. Article CAS PubMed Google Scholar * Salamone JD, Cousins MS, Bucher S (1994). Anhedonia or
anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. _Behav Brain Res_ 65: 221–229. Article CAS
PubMed Google Scholar * Salamone JD, Yohn SE, López-Cruz L, Miguel NS, Correa M (2016). Activational and effort-related aspects of motivation: neural mechanisms and implications for
psychopathology. _Brain_ 139: 1325–1347. Article PubMed PubMed Central Google Scholar * Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE (2007). The association between fatigue and
inflammatory marker levels in cancer patients: a quantitative review. _Brain Behav Immun_ 21: 413–427. Article CAS PubMed Google Scholar * Sinha N, Manohar S, Husain M (2013).
Impulsivity and apathy in Parkinson’s disease. _J Neuropsychol_ 7: 255–283. Article PubMed Google Scholar * Skvortsova V, Degos B, Welter M-L, Vidailhet M, Pessiglione M (2017). A
selective role for dopamine in learning to maximize reward but not to minimize effort: evidence from patients with parkinson's disease. _J Neurosci_ 37: 6087–6097. Article CAS PubMed
PubMed Central Google Scholar * Skvortsova V, Palminteri S, Pessiglione M (2014). Learning to minimize efforts versus maximizing rewards: computational principles and neural correlates.
_J Neurosci_ 34: 15621–15630. Article CAS PubMed PubMed Central Google Scholar * Smith LB, Leo MC, Anderson C, Wright TJ, Weymann KB, Wood LJ (2014). The role of IL-1_β_ and TNF-_α_
signaling in the genesis of cancer treatment related symptoms (CTRS): a study using cytokine receptor-deficient mice. _Brain Behav Immun_ 38: 66–76. Article CAS PubMed Google Scholar *
Starkstein SE, Pahissa J (2014). Apathy following traumatic brain injury. _Psychiatr Clin North Am_ 37: 103–112. Article PubMed Google Scholar * Stertz L, Magalhã PV, Vio Kapczinski F
(2013). Is bipolar disorder an inflammatory condition? The relevance of microglial activation. _Curr Opin Psychiatry_ 26: 19–26. Article PubMed Google Scholar * Swardfager W, Rosenblat
JD, Benlamri M, McIntyre RS (2016). Mapping inflammation onto mood: Inflammatory mediators of anhedonia. _Neurosci Biobehav Rev_ 64: 148–166. Article CAS PubMed Google Scholar * Toomey
R, Faraone SV, Simpson JC, Tsuang MT (1998). Negative, positive, and disorganized symptom dimensions in schizophrenia, major depression, and bipolar disorder. _J Nerv Ment Dis Issue_ 186:
470–476. Article CAS Google Scholar * Vichaya EG, Hunt SC, Dantzer R (2014). Lipopolysaccharide reduces incentive motivation while boosting preference for high reward in mice.
_Neuropsychopharmacology_ 39: 2884–2890. Article CAS PubMed PubMed Central Google Scholar * Wardle MC, Treadway MT, Mayo LM, Zald DH, de Wit H (2011). Amping up effort: effects of
d-amphetamine on human effort-based decision-making. _J Neurosci_ 31: 16597–16602. Article CAS PubMed PubMed Central Google Scholar * Wichers MC, Koek GH, Robaeys G, Praamstra AJ, Maes
M (2005). Early increase in vegetative symptoms predicts IFN-alpha-induced cognitive-depressive changes. _Psychol Med_ 35: 433–441. Article CAS PubMed Google Scholar * Wood LJ, Weymann K
Inflammation and neural signaling: etiologic mechanisms of the cancer treatment-related symptom cluster (2013) _Curr Opin Support Palliat Care_ 7: 54–59. Article PubMed PubMed Central
Google Scholar * Yohn SE, Arif Y, Haley A, Tripodi G, Baqi Y, Müller CE _et al_ (2016). Effort-related motivational effects of the pro-inflammatory cytokine interleukin-6: pharmacological
and neurochemical characterization. _Psychopharmacology_ 233: 3575–3586. Article CAS PubMed Google Scholar * Young JJ, Bruno D, Pomara N (2014). A review of the relationship between
proinflammatory cytokines and major depressive disorder. _J Affect Disord_ 169: 15–20. Article CAS PubMed Google Scholar * Yüksel C, Öngür D (2010). Magnetic resonance spectroscopy
studies of glutamate-related abnormalities in mood disorders. _Biol Psychiatry_ 68: 785–794. Article PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We would
like to acknowledge all members of the intensive care research department of the Radboud University Medical Center for the opportunity to perform this study and for all their practical help,
particularly medical student Noor Thijs. AUTHOR INFORMATION Author notes * Peter Pickkers, Masud Husain and Marieke E van der Schaaf: These authors contributed equally to this work. AUTHORS
AND AFFILIATIONS * Department of Experimental Psychology University of Oxford, Oxford, UK Amelia Draper, Matthew AJ Apps & Masud Husain * Department of Intensive Care Medicine, Radboud
University Medical Center, Nijmegen, The Netherlands Rebecca M Koch & Peter Pickkers * Department of Internal Medicine, Radboud University Medical Centre, Nijmegen, The Netherlands Jos
WM van der Meer * Donders Institute for Brain, Centre for Cognitive Neuroimaging, Cognition and Behaviour, Radboud University, Nijmegen, The Netherlands Marieke E van der Schaaf Authors *
Amelia Draper View author publications You can also search for this author inPubMed Google Scholar * Rebecca M Koch View author publications You can also search for this author inPubMed
Google Scholar * Jos WM van der Meer View author publications You can also search for this author inPubMed Google Scholar * Matthew AJ Apps View author publications You can also search for
this author inPubMed Google Scholar * Peter Pickkers View author publications You can also search for this author inPubMed Google Scholar * Masud Husain View author publications You can also
search for this author inPubMed Google Scholar * Marieke E van der Schaaf View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR
Correspondence to Marieke E van der Schaaf. ADDITIONAL INFORMATION Supplementary Information accompanies the paper on the Neuropsychopharmacology website SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION (DOCX 40 KB) POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 RIGHTS AND
PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s
Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the
license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Draper, A., Koch, R., van der Meer, J. _et al._ Effort but not Reward Sensitivity is Altered by Acute Sickness Induced by Experimental Endotoxemia in Humans. _Neuropsychopharmacol._ 43,
1107–1118 (2018). https://doi.org/10.1038/npp.2017.231 Download citation * Received: 13 December 2016 * Revised: 29 August 2017 * Accepted: 05 September 2017 * Published: 26 September 2017 *
Issue Date: April 2018 * DOI: https://doi.org/10.1038/npp.2017.231 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
:max_bytes(150000):strip_icc():focal(216x0:218x2)/benedict-cumberbatch-1-435-4-20cc736017b24435a3498a49d7c22b0e.jpg)