- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Traditionally, neutralising antibodies that are directed to the major surface glycoprotein hemagglutinin (HA) head domain are measured as surrogate correlates of protection against
influenza. In addition to neutralization, hemagglutinin-specific antibodies may provide protection by mediating antibody-dependent cellular cytotoxicity (ADCC). During the 2009 pandemic,
vaccination induced HA-specific antibodies that were mostly directed to the conserved HA stalk domain. However, the protective role of these antibodies has not been investigated in detail.
We quantified the HA head and stalk-specific antibodies, their avidity, ability to neutralise virus and activate natural killer cells in an ADCC assay. We analyzed sera obtained from 14
healthcare workers who had low hemagglutination inhibition (HI) antibody titres at 3 months after pandemic H1N1 vaccination as well as from 22 controls. Vaccination resulted in a HA stalk
dominant antibody response in both low responders and controls. Revaccination of low responders, 5 months later, resulted in a boost in antibodies, with HA head-specific antibodies
dominating the response. Comparative analysis of head and stalk antibody avidities revealed that stalk-specific antibodies were qualitatively superior. Furthermore, stalk-specific antibodies
mediated virus neutralization and had significantly higher ADCC activity than head-specific antibodies. Despite the head and stalk-specific antibodies being lower in low responders, they
had comparable antibody avidity, ADCC functionality and neutralising capacity to those of controls who had high HI titres post-vaccination. Thus, our study has demonstrated that HA
stalk-specific antibodies may have an important role in protection through neutralization and ADCC in low responders who do not maintain seroprotective HI antibodies. SIMILAR CONTENT BEING
VIEWED BY OTHERS PERSISTENTLY HIGH ANTIBODY RESPONSES AFTER AS03-ADJUVANTED H1N1PDM09 VACCINE: DISSECTING THE HA SPECIFIC ANTIBODY RESPONSE Article Open access 01 April 2021 IMMUNE RESPONSES
TO A SINGLE DOSE OF THE AZD1222/COVISHIELD VACCINE IN HEALTH CARE WORKERS Article Open access 29 July 2021 IMMUNOGENICITY EVALUATION AFTER BNT162B2 BOOSTER VACCINATION IN HEALTHCARE WORKERS
Article Open access 26 July 2022 INTRODUCTION Influenza pandemics occur at unpredictable intervals when a novel influenza virus arises which can place a major strain on the global
healthcare system. These pandemic viruses can cause high levels of severe illness and death. In 2009, an influenza A H1N1 virus strain caused a pandemic that started in Mexico and California
then rapidly spread globally. The pandemic H1N1 (H1N1pdm) strain was antigenically distinct from the recently circulating seasonal H1N1 strains and the majority of the population was
immunologically naïve to this virus. Annual influenza vaccination is recommended for healthcare workers (HCW) so as to maintain the integrity of the healthcare system, reduce absenteeism and
reduce influenza A transmission to vulnerable patients.1 Vaccination of HCWs has been shown to protect hospitalised patients as well as decrease influenza-like illness and mortality in
residents of care-facilities.2 During the 2009 pandemic outbreak, the World Health Organization prioritised HCW for vaccination. H1N1pdm vaccination studies showed that a single dose of
pandemic vaccine elicited protective serum hemagglutination inhibition (HI) titres in adults, including HCW.3–8 However, seasonal influenza vaccines did not induce protection against the
novel H1N1pdm virus.9,10 HI antibodies are directed to the major surface glycoprotein, hemagglutinin (HA), and are the primary correlate of protection. HA is synthesised as a precursor, HA0,
which is then cleaved by host proteases into disulphide-linked HA1 and HA2 subunits, activating virus infectivity.11 Antibodies directed to the HA head domain that is composed of the
majority of the HA1 subunit prevent virus attachment to the sialic acids on host cells. These antibodies directed to the immunodominant head of the HA have potent neutralising activity that
can be detected by HI or microneutralization assays. Antibodies directed to the HA stalk domain, primarily composed of HA2 subunit and the N- and C-terminal ends of HA1, have other
functions, including blocking viral fusion with the host cell and antibody-dependent cellular cytotoxicity (ADCC).12 H1N1pdm vaccines preferentially induced HA stalk-specific antibodies. In
contrast, seasonal inactivated vaccines induce strain specific antibodies directed to the HA head domain and minimal HA stalk-specific antibodies.13,14 Furthermore, HA stalk antibodies are
postulated to be boosted most efficiently in individuals previously exposed to HAs whose head domains differ substantially from the infecting novel virus strain. Here, a memory B-cell
response is boosted against the conserved HA stalk domain.15 Importantly, HA stalk-specific antibodies are broadly reactive and may have a significant role in protection against infection in
the absence of HA head-specific antibodies. In this study, we analyzed the magnitude of HA-specific antibodies induced after adjuvanted pandemic influenza vaccination in HCW. We also
analyzed the quality as well as the neutralising and ADCC function of HA-specific antibodies in low-responder HCW who fail to maintain seroprotective HI responses after H1N1pdm vaccination.
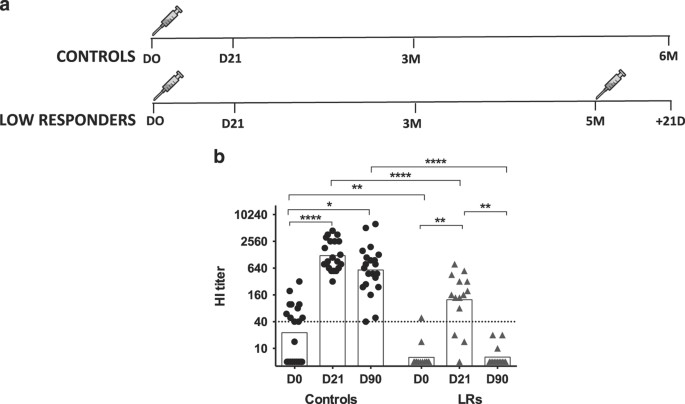
RESULTS LOW RESPONDERS FAILED TO MAINTAIN HI TITRES POST-VACCINATION Thirty-six HCW were recruited to the study based on their HI response and split into two groups; low responders (LRs) who
failed to maintain protective HI titres by 3 months (3M) and a control group (Figure 1a). Fifty per cent of the controls had protective HI titres (geometric mean titre (GMT)=23) before
vaccination in comparison to 1 LR (7%) (GMT=6). Following pandemic H1N1 vaccination, HI titres increased significantly by D21 in both groups (_P_<0.01). However, in LRs, titres were
significantly lower (GMT=132) (_P_<0.001) than controls (GMT=1,223). All the controls maintained their protective HI titres at 3 mol/l, whereas HI titres decreased below protective levels
for all the LRs (Figure 1b). This decrease may imply that LRs are no longer protected, although their antibodies may mediate protection through other mechanisms. THE BACK-BOOST OF
CROSS-REACTIVE ANTIBODIES TO PRE-PANDEMIC SEASONAL H1N1 STRAINS IS LOWER IN LRS The H1N1 strains circulated from 1977 to 2008, when the H1N1pdm appeared and replaced them as the seasonal
strain. We examined the cross-reactivity of the HI response in 20 controls and 14 LRs to the following historical strains; USSR/77, Brazil/78, Taiwan/86, Texas/91, New Caledonia/99 and
Brisbane/07 (Figure 2). Interestingly, pre-vaccination, LRs had significantly lower titres (GMTs<40) to all the seasonal H1N1 strains tested in this study (_P_<0.05) than the
equivalent responses to A/California/07/09. This was in contrast to controls who had HI GMT>40 for all strains except for the oldest strains (USSR/77 and Brazil/78). Of the controls,
70–100% had seropositive pre-vaccination titres to Taiwan/86, Texas/91, New Caledonia/99 and Brisbane/07. Pre-vaccination sera showed greatest reactivity to Texas/91 in both controls and LRs
with GMT of 137 and 40, respectively. Pre-vaccination titres to Texas/91 were higher than those observed to 2009 H1N1pdm. After pandemic H1N1 vaccination, (D21) sera cross-reacted with all
the seasonal H1N1 strains in controls with seroprotective titres in 80–100% of subjects. Back-boosting, with HI titres greater or comparable to those to H1N1pdm, was induced for Texas/91 and
Taiwan/86 with GMTs of 1,841 and 618, respectively. In comparison to controls, cross-reactive antibodies in LRs were significantly lower to 4 of the 6 strains; USSR/77, Brazil/78, Taiwan/86
and Texas/91 (_P_<0.05). At D21, the GMTs to USSR/77 and Brazil/78 were low at 23 and 33, respectively, whereas for the 4 other seasonal H1N1 strains titres were >40 (range: 69–442).
Seroprotection was lower in LRs (42–92%) compared to controls, with the highest seroprotective titres observed for the Texas/91 strain. In summary, these results show that H1N1
cross-reactivity of post-H1N1pdm vaccination sera to seasonal H1N1 strains is reduced in LRs. HA STALK-SPECIFIC IGG ANTIBODIES DOMINATE THE RESPONSE TO PANDEMIC H1N1 We dissected the
specificity of the antibodies to the different HA domains, which may have different functions in controlling infection. Sera were evaluated for antibodies specific for the H1N1pdm whole HA,
HA1 (head domain) and chimeric HA construct, cH6/1 (stalk domain). Pre-vaccination, antibodies specific to the whole HA were significantly lower in LRs compared to controls (_P_<0.05).
Vaccination resulted in an increase in these antibodies, however to a lesser magnitude than in controls (Figure 3a). The antibody levels induced by vaccination were significantly lower in
LRs compared to controls to both the HA head and the stalk domains. In controls, both the HA head and stalk-specific antibodies increased significantly after vaccination (_P_<0.01).
However, in the LRs only stalk-specific antibodies increased significantly (_P_<0.001), whereas HA head-specific antibodies remained low. The fold increase in HA stalk-specific antibodies
was comparable between the two groups, whereas controls had a higher fold increase in HA head-specific antibodies (_P_<0.01) (Figure 3b). HA stalk-specific antibodies dominated the
response pre- and post-vaccination in both groups (Figure 3c). There was a significant increase in the proportion of HA stalk antibodies at D21 in comparison to pre-vaccination ratios in LRs
(_P_<0.05). Pre-vaccination, 75% of controls had HA stalk dominant IgG antibodies, whereas at D21, 82% had a HA stalk dominant IgG antibodies. However, in LRs, there was an increase from
57 to 85% in number of participants with stalk dominant antibody responses at D0 and D21, respectively (Figure 3c). REVACCINATION OF LRS BOOSTS THE HA HEAD-SPECIFIC ANTIBODIES Next, we
analysed the kinetics of the HA-specific IgG antibodies in LRs following revaccination with the AS03 adjuvanted monovalent pandemic H1N1 vaccine. After revaccination, the HA head was no
longer novel so we expected a boost of the response to HA head domain. As we did not collect sera from controls at the time of LR revaccination (5M), we used sera collected at 6M for
comparison purposes. IgG antibodies specific to the whole HA where maintained for at least 6M in controls but not in LRs where they decreased to pre-vaccination levels. However,
revaccination of LRs resulted in a boost in the whole HA-specific antibodies (_P_<0.01) (Figure 4a). In controls, the HA head-specific IgG antibodies decreased to pre-vaccination levels
by 6M. Similarly, in LRs, HA head-specific IgG antibodies returned to pre-vaccination levels at the time of revaccination (5M). The level of these HA1 specific IgG antibodies was
significantly lower in the LR group at 5M (_P_<0.001) (Figure 4b). In contrast to HA head-specific antibodies that decreased by 6M post-vaccination, HA stalk-specific antibodies were
maintained for at least 6M in controls. However, in LRs, HA stalk-specific antibodies were significantly lower than controls (_P_<0.001) and decreased to pre-vaccination levels 5M
post-vaccination (Figure 4c). Revaccination boosted both the HA head and HA stalk-specific IgG response by D21 (_P_<0.01). After the first vaccination, the IgG titres increased mostly to
the HA stalk, in contrast revaccination induced antibody responses mostly to the HA head (Figure 4d). Twenty-one days after the first vaccination, there was higher fold increase in HA
stalk-specific IgG than HA head-specific IgG titres. However, following revaccination, there was a significantly higher fold increase in IgG titres to the HA head than after the first
vaccination (_P_<0.001). HA STALK-SPECIFIC IGG ANTIBODIES SHOW AVIDITY SUPERIOR TO HA HEAD-SPECIFIC ANTIBODIES To evaluate the quality of the antibodies in the controls and LRs, we
measured the avidity of HA head and HA stalk-specific IgG antibodies in these two cohorts of HCW. The avidity of HA-specific IgG antibodies was measured in an avidity enzyme-linked
immunosorbent assay (ELISA) using NaSCN as a chaotropic agent. Untreated sera were compared to those treated with 1.5 mol/l NaSCN, and the percentage of bound IgG antibodies remaining after
1.5 mol/l NaSCN treatment was calculated. At baseline, the avidity of head-specific antibodies was low. The avidity of head-specific antibodies increased significantly in both controls and
LRs following vaccination (_P_<0.05) (Figure 5a). In controls, the avidity index increased from a mean of 3.02–7.11% at D21. In LRs, an increase from a mean of 1.95–8.14% was observed
post-vaccination. The avidity of HA stalk-specific antibodies was significantly higher than that of HA head-specific antibodies at both time-points (Figure 5b). In controls, the % of IgG
antibodies remaining bound after NaSCN treatment was 25.74% (range: 5.3–69%) at baseline. Vaccination resulted in a significant increase in the avidity of HA stalk-specific IgG antibodies in
LRs only. The avidity index of the IgG antibodies increased from 25.40% (range: 7.46–55.92%) at baseline to 30.82% (range: 17.13–65.26%) at D21 post-vaccination in LRs (_P_<0.01).
Despite, the quantity of the stalk-specific antibodies being lower in LRs compared to controls, the antibodies displayed equivalent avidity at both time-points. HA STALK-SPECIFIC ANTIBODIES
CAN NEUTRALISE VIRUS IN THE ABSENCE OF HI ANTIBODIES To assess the _in vitro_ functionality of H1 HA stalk-specific antibodies, we performed a microneutralization assay with a virus that
expresses a cH9/1 HA and an irrelevant N3 neuraminidase. The HA stalk domain of this virus is derived from H1, and the HA head domain is from H9. Subjects in this study are expected to be
naive to the avian H9 and N3 in this virus. No standard virus neutralization titre for 50% protection has been recognised. However, a previous study in H1N1pdm-infected adults showed that
the MN titre was generally twofold higher than the HI titre when the HI titre was ⩽160.16 We therefore used a threshold of 80 to define protective titres. H1 HA stalk-specific neutralising
antibodies were detected pre-vaccination when the majority of controls (50%) and LRs (93%) had HI titres<40 (Figure 6a). These H1 HA stalk-specific neutralising antibodies were present in
all subjects with GMT of 204 and 171.5 in controls and LRs, respectively. Vaccination resulted in a significant increase in HA stalk-specific neutralising antibodies in LR (_P_<0.05) but
not in controls. HA STALK-SPECIFIC ANTIBODIES ARE BETTER MEDIATORS OF NATURAL KILLER (NK) CELL ACTIVATION As the HA-specific antibody response was dominated by HA stalk-specific antibodies,
we wanted to determine the functionality and possible protective mechanisms of these antibodies, especially in LRs where HA head-specific antibodies were low. We therefore assessed the ADCC
induced by HA head and HA stalk-specific antibodies using an NK activation assay measuring CD107a (degranulation) and INF-γ expression by flow cytometry. The gating strategy for flow
cytometry is shown in Supplementary Figure S1. We used sera diluted 1:10 rather than endpoint titres as a positive correlation between NK cell activation at a single sera dilution of 1:10
and NK activation end point titre has been previously shown.17 Pre-vaccination, NK cell activation was mediated by both HA head and HA stalk-specific antibodies and the levels of NK cell
activation were comparable between LRs and controls. Vaccination resulted in increased NK cell activation with a significant increase in head and stalk antibody-mediated CD107a expression
being observed for both LRs and controls (_P_<0.01)(Figure 6b,c). However, the levels of INF-γ expression mediated by HA head-specific antibodies did not increase for either LRs or
controls. In contrast to controls who had an increase in INF-γ expression at D21, no change in HA stalk antibody-mediated INF-γ expression was detected in LRs (Figure 6c). In order to make a
direct comparison of the levels of head versus stalk antibody-mediated NK cell activation, we standardized sera in an ELISA assay to give an optical density (OD) of 0.7±0.2. In controls,
pre-vaccination HA head and HA stalk-specific antibodies induced similar NK cell expression of INF-γ (Figure 6d). However, post-vaccination, HA stalk-specific antibodies induced
significantly higher INF-γ expression than head-specific antibodies in controls (_P_<0.01). In contrast, LRs’ HA stalk-specific antibodies induced significantly higher INF-γ expression at
D0 than head-specific antibodies (_P_<0.05), whereas at D21 the INF-γ expression levels were maintained. Pre-vaccination, HA stalk-specific antibodies induced significantly higher NK
cell CD107a expression than HA head-specific antibodies in both LRs and controls (Figure 6e). Post-vaccination, only HA head-specific antibodies from LRs had an increased ADCC activity as
measured by CD107a expression. Despite no increase in CD107a expression at D21, the HA stalk-specific antibodies maintained a significantly higher ADCC induction than HA head-specific
antibodies in both LRs and controls (_P_<0.01). Furthermore, there was no significant difference in the ADCC mediated by both head and stalk-specific antibodies between LRs and controls.
Interestingly, there was no correlation between NK cell activation and HI titres in controls (data not shown). However, in LRs, HI titres negatively correlated with NK cell INF-γ expression
mediated by both HA head and HA stalk antibodies (_P_<0.01). This suggests that H1N1pdm vaccination induced HA-specific antibodies that can mediate FcγR-dependent NK cell activation,
regardless of HI antibodies. DISCUSSION The H1N1pdm virus contained a novel HA head domain that was different from the pre-pandemic seasonal H1 viruses. Pandemic vaccination induced
antibodies that were directed towards the immunosubdominant conserved epitopes on the HA stalk domain.13,14 In the current study, we aimed to investigate and to increase understanding of the
HA-specific IgG responses of LR HCW following 2009 pandemic influenza A (H1N1) vaccination. We used chimeric HA constructs to differentiate between the response to the HA head and stalk
domains as these antibodies may have different functions in controlling influenza infection. Influenza specific responses are commonly measured by the HI assay. HI antibodies are a correlate
of protection, and are mostly directed to the HA head domain and do not necessarily reflect the entire spectrum of vaccine induced antibodies. We demonstrated that LRs IgG antibodies were
quantitatively inferior to controls but qualitatively similar. We showed that a single dose of AS03 adjuvanted pandemic H1N1 vaccine elicited a significant HI response in controls that was
maintained by 3M post-vaccination, whereas in LRs the response was lower and waned by 3M. The lower HI response may be attributed to the lower quantities of HA head-specific antibodies in
LRs as antibodies measured by the HI assay are predominantly HA head-specific. Back-boosting, where a substantial response to older viruses is induced, depending on pre-exposure has been
recently described for both H3N2 and H1N1 viruses.18,19 Li _et al._18 showed sera from H1N1pdm-infected people had considerable cross-reactivity with H1N1 strains from 1984 to 1994. They
showed that the back-boost for H1N1 was due to a shared epitope in the head domain at H1N1pdm and most seasonal H1N1 strains from 1983 to 1996. HAs of most seasonal H1N1 between 1983 and
1996 contained a K133 amino acid at Sa antigenic site of HA, but not H1N1 viruses before 1983 or after 1996. Our results are in agreement with this as we showed back-boost of HI against
viruses up to Texas/91 but not much against NC/99 and Brisbane/07. LRs had a lower back-boost of cross-reactive HI antibodies to pre-pandemic, seasonal H1N1 strains. This may be due to
differences in exposure history to different H1N1 strains or due to limited induction of HI antibodies that bind to shared epitopes on the HA of pandemic and seasonal H1N1 strains. Moreover,
the pandemic vaccine may have elicited antibodies with broader specificities that bind the same epitopes as antibodies induced by seasonal H1N1 strains as well as to additional epitopes.
Despite the lower HI antibodies detected in the LR cohort, HA stalk-specific antibodies may also provide an alternative method of protection. We tested for the HA domain binding of IgG
antibodies and found that HA stalk-specific antibodies were lower pre-vaccination in LRs, possibly due to differences in the priming or infection history between controls and LRs or due to
the characteristics of the HA-specific memory previously generated. Even though pre-vaccination HA stalk-specific antibodies were lower, a single dose of H1N1pdm vaccine elicited significant
stalk-specific antibodies more efficiently than HA head-specific antibodies. However, 5M post-vaccination, HA head and stalk-specific antibodies in LRs had decreased and were significantly
lower than those in controls. Although none of the HCW reported H1N1pdm infection, it cannot be ruled out that some controls had exposure or subclinical infection between the 5M and 6M
interval. Alternatively, the lower antibodies in LR could be explained by poor antibody maintenance in this cohort. The decreased antibody titres may not necessarily mean that the HCW were
no longer protected, as factors other than antibody titres may be important in long-term protection. These findings raise questions as to: (i) why the conserved HA stalk domain shows
superior immunogenicity after H1N1pdm vaccination, whereas after seasonal vaccination the HA head-specific response dominates20,21; and (ii) the effect of homologous boosting on HA domain
binding. We found a significantly higher boost in head than stalk-specific antibodies following revaccination of LRs. This is in line with previous reports following H1N1pdm and H5N1
vaccination.22,23 Ellebedy _et al._ demonstrated that the first dose of H5N1 vaccination elicited a stronger stalk-specific response than head-specific response. However, booster vaccination
resulted in a vigorous head-specific response and marginal increase in stalk-specific antibodies.23 These findings support the theory that at the time of the first vaccination, vaccinees
had negligible pre-existing memory B cells specific to the immunodominant HA head domain in comparison with stalk-specific ones. The strong stalk-specific antibody response likely reflects
the reactivation of stalk-specific memory B cells generated by previous seasonal H1N1 infections. Accordingly, memory B cells specific for the immunosubdominant HA stalk were recruited and
reactivated in the absence of competition from memory B cells specific for the immunodominant HA head domain. A primary antibody response was also induced to the HA head domain resulting in
the increase in plasmablasts and memory B cells generation. Upon booster vaccination, the recently generated head-specific memory B cells out-competed the stalk-specific memory B cells.24
The quality of antibodies is important for their functionality. In our analysis, HA stalk-specific antibodies not only dominated the response but also displayed superior avidity to
head-specific antibodies. These results are in agreement with the findings of He _et al._,20 who showed that plasmablast-derived polyclonal antibodies from elderly (>70 years) after
H1N1pdm vaccination had higher avidity than those from young (18–32 years) and that HA2 specific antibodies had higher avidity than HA1 specific antibodies. The higher avidity of
stalk-specific antibodies could be explained by antibody secreting cells producing these antibodies being derivatives of memory B cells from previous H1N1 encounters that have gone through
several rounds of selection and affinity maturation.25,26As the 2009 pandemic H1N1 contained a novel head, different from the pre-pandemic seasonal H1N1 viruses, most of the HA head-specific
antibodies would have been generated from antibody secreting cells from a primary immune response that contain few somatic mutations, explaining the lower avidity of HA head-specific
antibodies. It has been shown that broadly cross-reactive HA-specific antibodies that exhibit high levels of somatic hypermutation are induced after H1N1pdm vaccination or infection.13,27
Analysis of the somatic mutation status of the immunoglobulin genes from the memory B cells or plasmablasts specific for the HA head and stalk domains is required to give insight into the
origin of the response in our cohort of HCW. These high-avidity HA stalk-specific antibodies may have an important role in protecting these LRs in the absence of HI antibodies. However, a
correlate of protection for these antibodies remains to be determined. We showed that high titres neutralising HA stalk-specific antibodies were present in all subjects pre-vaccination, when
HI antibodies were absent or low in the majority of the HCW. The neutralising stalk-specific antibodies were maintained in controls and only increased significantly in LRs after
vaccination. These neutralising stalk-specific antibodies could be protective regardless of the HI titre and could have a significant role in protecting the low responders who have
significant quantities of HA stalk-specific antibodies. In addition to virus neutralization, HA-specific antibodies can bind to infected cells and activate NK cells through FcγR resulting in
lysis of target cells and secretion of cytokines like INF-γ.17 In this study, we showed that ADCC antibodies were present pre-vaccination when the majority of the subjects were HI
seronegative and these ADCC antibodies were mostly directed towards the HA stalk domain. These pre-existing ADCC antibodies may assist in clearance of H1N1pdm virus infection.
Post-vaccination, the HA stalk-specific antibodies were better mediators of ADCC than HA head-specific antibodies. This difference in the ADCC activity between HA head and stalk-specific
antibodies may be attributed to the relative difference in affinities of these antibodies for HA. Despite LRs having lower post-vaccination HI titres and lower HA-specific IgG titres than
controls; their antibodies had comparable ability to mediate NK cell activation. We found no correlation between HI titre and NK cell activation in controls, whereas in LRs INF-γ expression
negatively correlated with HI titres. Our results are similar to a previous report that found no correlation between HI titres and HA-specific ADCC antibodies.17 However, these authors
characterised the response induced by antibodies that bound to the whole HA protein, whereas we dissected between HA head and stalk-specific antibodies. Of note, the HI and ADCC assays
measure different aspects of antibody function. HI assay assesses the binding of the antibody through its Fab region to antigen, whereas the ADCC assay assesses the NK activation by
antibodies bound through their Fc region. Fc–FcγR interactions are required for the protection by HA stalk-specific antibodies suggesting a role of ADCC antibodies in protection.28–30
Furthermore, cross-reactive ADCC antibodies to influenza virus have been reported in the absence of neutralising antibodies.31 Thus, it is likely that HA stalk-specific antibodies may
provide alternative protection in this cohort of LRs where HA head-specific antibodies are low and induced short-term protection as measured by the HI assay. This underlines a possible
limitation of using HI titre as a sole predictive correlate of protection. In summary, the characterisation of the HA stalk-specific antibodies denotes an important step in understanding the
protective capacity of these antibodies. Further analysis of the HA stalk-specific antibody repertoire and function would facilitate rational vaccine design. In LRs where the HI responses
are poor, vaccination strategies could potentially aim to induce HA stalk-specific antibodies. The lower quantity of HA-specific antibodies could be compensated for by the higher avidity of
HA stalk-specific antibodies that have better ADCC functionality as well as neutralising capacity. Enhancing the amount of HA stalk-specific antibodies elicited by vaccination or booster
vaccination should be considered in LR HCW. These antibodies could provide broad cross-protection and could be used for immunological priming of the general population to quickly respond to
a future pandemic influenza threat. MATERIALS AND METHODS STUDY DESIGN AND PARTICIPANTS The HCWs were vaccinated in October 2009 at the Haukeland University Hospital (HUH, Bergen, Norway)
with a single dose of the monovalent pandemic H1N1 vaccine (Pandemrix) adjuvanted AS03 (GlaxoSmithKline (GSK), Wavre, Belgium). Thirty-six HCW were retrospectively selected by their HI
antibody response at 3M after pandemic H1N1 vaccination. On this basis, a cohort of 14 LRs who failed to respond or maintain a protective HI antibody response (titre⩾40) at 3M after
vaccination were selected. As a control group, 22 HCW who maintained a protective HI antibody response were selected. LRs were offered a second dose of vaccine, and 12 participants were
revaccinated 5M later. Sera were collected at vaccination, day 21 (D21), 3M, and 6M post-vaccination in controls. In revaccinated LRs, additional sera were collected at revaccination (5M)
and 21 days later (Figure 1a). The inclusion and exclusion criteria for this study are described elsewhere.7 All participants provided written informed consent before inclusion in the study,
which had ethical and regulatory approval (ClinicalTrials.gov NOT01003288). HI ASSAY Serum samples were treated with receptor destroying enzyme and run in the HI assay twice in duplicate
using turkey red blood cells as previously described.7 HI responses were analyzed against the homologous pandemic H1N1 virus strain, A/California/07/09, and against six prototype seasonal
H1N1 strains; A/USSR/90/77, A/Brazil/11/78, A/Taiwan/1/86, A/Texas/36/91, A/New Caledonia/20/99 and A/Brisbane/59/07. Seroprotection was defined as an HI titre ⩾40. Titres<10 were
assigned a value of 5 for calculation purposes. ANTI-HA IGG ELISA Sera were evaluated in duplicate for IgG antibodies.32 The plates were coated with influenza whole HA, HA1
(A/California/06/2009(H1N1)) hexahistidine-tagged (eEnzyme, IA-01SW-005P) or cH6/1, a chimeric HA (cHA) that combines H1 stalk domain with globular head domain derived from H6 influenza A
virus.33 The antibody concentrations were calculated as endpoint titres that were determined when the reactivity of the diluted sample reached background levels. ANTI-HA IGG AVIDITY ELISA
Sera were evaluated for avidity of antibodies against influenza HA1 (A/California/06/2009(H1N1)) hexahistidine-tagged (eEnzyme, Gaithersburg, MD, USA) and cH6/1.34 Sera were first diluted in
duplicate to the appropriate OD of 0.7±0.3 in a direct ELISA and 1.5 mol/l sodium thiocyanate (NaSCN) (Sigma, St Louis, MO, USA) was added 1 h after the sera, followed by 1 h incubation.
The avidity index calculated as: (OD450 treated serum/OD450 untreated serum)×100%. VIRUS NEUTRALIZATION ASSAY EXPANSION OF MDCK CELLS Madin-Darby Canine Kidney (MDCK) (ATCC, Manassas, VA,
USA, CCL-34) were cultivated in DMEM supplemented with 10% fetal bovine serum (FBS) and 1× Penicillin/Streptomycin. MDCK cells in log-phase growth were plated in 96-well plates such that
they are 70–90% confluent at the time of inoculation. Serum samples were heat-inactivated at 56 °C for 30 min and run in duplicate. The serum samples were then diluted 2 fold in virus growth
medium containing Dulbecco’s Modified Eagle’s Medium with tosyl phenylalanyl chloromethyl ketone-trypsin, 0.14% bovine serum albumin, 100 units/ml penicillin, 100 μg/ml streptomycin and
0.25 μg/ml amphotericin B. Chimeric 9/1N3 virus was diluted to 50% tissue culture infectious dose (TCID50) of 100 per 50 μl in virus growth medium. Fifty microliters of diluted sera was
incubated with 50 μl of virus for 1 h at 37 °C. MDCK cells were washed once with phosphate-buffered solution (PBS) and 100 μl of serum-virus mixture was added to the cells. Cells were
incubated at 37 °C for 1 h then washed once with PBS before 50 μl of diluted serum and 50 μl of virus growth medium were added to each well. Infected MDCK cells were incubated for 72 h at 37
°C. Fifty microliters of the supernatant was transferred to a 96-well V bottom plate and 50 μl of 0.7% turkey red blood cells added. Hemagglutination activity was measured to detect the
endpoint of agglutination. ADCC NK CELL ACTIVATION ASSAY The ADCC assay measuring intracellular NK cell IFNγ and CD107a expression was conducted as previously described with minor
modifications.35 Briefly, 96-well plates were coated overnight at 4 °C with 1 μg/ml HA1 (A/California/06/2009(H1N1)) 6×His tagged (eEnzyme, USA) or chimeric cH6/1 in PBS. Plates were washed
with PBS and incubated with heat-inactivated human sera for 2 h at 37 °C. After washing, 105 CD16 176v NK-92 cells (mycoplasma-free, human NK cell line expressing high affinity 176V variant
CD16 receptor) (kindly provided by Fox Chase Cancer Center, Philadelphia, PA, USA) were added per test well. As a negative control for each sample, NK-92 cells (lacking expression of CD16)
were added to an additional well. The cells were incubated for 16 h at 37 °C with anti-CD107a-AF488 antibody (Biolegend, San Diego, CA, USA, 328610), Brefeldin A (5 μg/ml, BD) and monensin
(5 μg/ml, BD). Cells were stained with LIVE/DEAD Fixable Aqua dead cell staining kit (Invitrogen, Carlsbad, CA, USA), anti-CD3-PE CF594 (BD, Franklin Lakes, NJ, USA, 562280) and
anti-CD56-APC (BD, 555518) before intracellular staining with anti-IFN-γ-BV-421 (Biolegend, 502532). Cells were acquired on BD Fortessa (San Jose, CA, USA). Data analysis was done using
FlowJo version 10 (treeStar, Ashland, OR, USA). STATISTICS Two-tailed unpaired nonparametric Mann–Whitney tests and paired nonparametric Wilcoxon tests were performed using GraphPad Prism 6
(Graphpad, La Jolla, CA, USA). A _P_-value<0.05 was considered statistically significant. REFERENCES * Poland, G. A., Tosh, P. & Jacobson, R. M. Requiring influenza vaccination for
health care workers: seven truths we must accept. _Vaccine_ 23, 2251–2255 (2005). Article Google Scholar * Lemaitre, M. et al. Effect of influenza vaccination of nursing home staff on
mortality of residents: a cluster-randomized trial. _J. Am. Geriatr. Soc._ 57, 1580–1586 (2009). Article Google Scholar * Roman, F. et al. Effect on cellular and humoral immune responses
of the AS03 adjuvant system in an A/H1N1/2009 influenza virus vaccine administered to adults during two randomized controlled trials. _Clin. Vaccine Immunol._ 18, 835 (2011). Article CAS
Google Scholar * Nicholson, K. G. et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential
vaccines against H5N1 influenza. _Lancet_ 357, 1937–1943 (2001). Article CAS Google Scholar * Clark, T. W. et al. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. _N.
Engl. J. Med._ 361, 2424 (2009). Article CAS Google Scholar * Zhou, Y. et al. Seroprevalence of pandemic H1N1 antibody among health care workers in Hong Kong following receipt of
monovalent 2009 H1N1 influenza vaccine. _PLoS ONE_ 6, e27169 (2011). Article CAS Google Scholar * Madhun, A. S. et al. An adjuvanted pandemic influenza H1N1 vaccine provides early and
long term protection in health care workers. _Vaccine_ 29, 266–273 (2010). Article CAS Google Scholar * Greenberg, M. E. et al. Response to a monovalent 2009 influenza A (H1N1) vaccine.
_N. Engl. J. Med._ 361, 2405–2413 (2009). Article CAS Google Scholar * Centers for Disease Control and Prevention. Serum cross-reactive antibody response to a novel influenza A (H1N1)
virus after vaccination with seasonal influenza vaccine. _MMWR Morb. Mortal. Wkly. Rep._ 58, 521–524 (2009). Google Scholar * Hancock, K. et al. Cross-reactive antibody responses to the
2009 pandemic H1N1 influenza virus. _N. Engl. J. Med._ 361, 1945–1952 (2009). Article CAS Google Scholar * Lazarowitz, S. G. & Choppin, P. W. Enhancement of the infectivity of
influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. _Virology_ 68, 440–454 (1975). Article CAS Google Scholar * Krammer, F. & Palese, P. Universal
influenza virus vaccines: need for clinical trials. _Nat. Immunol._ 15, 3–5 (2014). Article CAS Google Scholar * Wrammert, J. et al. Broadly cross-reactive antibodies dominate the human B
cell response against 2009 pandemic H1N1 influenza virus infection. _J. Exp. Med._ 208 181 (2011). Google Scholar * Pica, N. et al. Hemagglutinin stalk antibodies elicited by the 2009
pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. _Proc. Natl Acad. Sci. USA_ 109, 2573–2578 (2012). Article CAS Google Scholar * Palese, P. & Wang,
T. T. Why do influenza virus subtypes die out? A hypothesis. _Mbio_ 2 (2011). * Veguilla, V. et al. Sensitivity and specificity of serologic assays for detection of human infection with
2009 pandemic H1N1 virus in U.S. populations. _J. Clin. Microbiol._ 49, 2210–2215 (2011). Article Google Scholar * Jegaskanda, S. et al. Age-associated cross-reactive antibody-dependent
cellular cytotoxicity toward 2009 pandemic influenza A virus subtype H1N1. _J. Infect. Dis._ 208, 1051–1061 (2013). Article CAS Google Scholar * Li, Y. et al. Immune history shapes
specificity of pandemic H1N1 influenza antibody responses. _J. Exp. Med._ 210, 1493–1500 (2013). Article CAS Google Scholar * Fonville, J. M. et al. Antibody landscapes after influenza
virus infection or vaccination. _Science_ 346, 996–1000 (2014). Article CAS Google Scholar * He, X. S. et al. Heterovariant cross-reactive B-cell responses induced by the 2009 pandemic
influenza virus A subtype H1N1 vaccine. _J. Infect. Dis._ 207, 288–296 (2013). Article CAS Google Scholar * Corti, D. et al. Heterosubtypic neutralizing antibodies are produced by
individuals immunized with a seasonal influenza vaccine. _J. Clin. Invest._ 120, 1663 (2010). Article CAS Google Scholar * Sangster, M. Y. et al. B cell response and hemagglutinin
stalk-reactive antibody production in different age cohorts following 2009 H1N1 influenza virus vaccination. _Clin. Vaccine Immunol._ 20, 867–876 (2013). Article CAS Google Scholar *
Ellebedy, A. H. et al. Induction of broadly cross-reactive antibody responses to the influenza HA stem region following H5N1 vaccination in humans. _Proc. Natl Acad. Sci. USA_ 111,
13133–13138 (2014). Article CAS Google Scholar * Li, G. M. et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells.
_Proc. Natl Acad. Sci. USA_ 109, 9047–9052 (2012). Article CAS Google Scholar * Khurana, S., Frasca, D., Blomberg, B. & Golding, H. AID activity in B cells strongly correlates with
polyclonal antibody affinity maturation _in-vivo_ following pandemic 2009-H1N1 vaccination in humans. _Plos Pathog._ 8, e1002920 (2012). Article CAS Google Scholar * Wrammert, J. et al.
Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. _Nature_ 453, 667 (2008). Article CAS Google Scholar * Li, G. M. et al. Pandemic H1N1 influenza vaccine
induces a recall response in humans that favors broadly cross-reactive memory B cells. _Proc. Natl Acad. Sci. USA_ 109, 9047–9052 (2012). Article CAS Google Scholar * DiLillo, D. J.,
Tan, G. S., Palese, P. & Ravetch, J. V. Broadly neutralizing hemagglutinin stalk-specific antibodies require Fcgamma R interactions for protection against influenza virus _in vivo_.
_Nat. Med._ 20, 143–151 (2014). Article CAS Google Scholar * Corti, D. et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A
hemagglutinins. _Science_ 333, 850–856 (2011). Article CAS Google Scholar * Tan, G. S. et al. A pan-H1 anti-hemagglutinin monoclonal antibody with potent broad-spectrum efficacy _in
vivo_. _J. Virol._ 86, 6179–6188 (2012). Article CAS Google Scholar * Jegaskanda, S. et al. Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity in intravenous
immunoglobulin as a potential therapeutic against emerging influenza viruses. _J. Infect. Dis._ 210, 1811–1822 (2014). Article CAS Google Scholar * Cox, R. J. et al. An early humoral
immune response in peripheral blood following parenteral inactivated influenza vaccination. _Vaccine_ 12, 993 (1994). Article CAS Google Scholar * Krammer, F., Pica, N., Hai, R., Margine,
I. & Palese, P. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. _J. Virol._ 87, 6542–6550 (2013). Article CAS Google
Scholar * Pedersen, G. K. et al. Serum IgG titres, but not avidity, correlates with neutralizing antibody response after H5N1 vaccination. _Vaccine_ 32, 4550–4557 (2014). Article CAS
Google Scholar * Jegaskanda, S. et al. Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity antibodies in the absence of neutralizing antibodies. _J. Immunol._ 190,
1837 (2013). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank the Healthcare workers at Haukeland University Hospital for participating in this study. We also
thank Dr Per Espen Akslesen and study nurses at the Bergen Clinical Vaccine Consortium, Haukeland University Hospital; Marianne Sævik, Hanne Søyland and Astrid Rykkje Heien. The study was
funded by the Influenza Centre at the University of Bergen and through funding from the Norwegian Directorate of Health. The Influenza Centre is funded by the Ministry of Health and Care
Services, Norway, the Norwegian Research Council Globvac programme (220670/H10), the European Union (Univax 601738), Helse Vest and the K.G. Jebsen Centre for Influenza Vaccines. The funders
had no role in study design, data collection and interpretation, or the decision to submit the work for publication. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Clinical
Science, The Influenza Centre, University of Bergen, Bergen, Norway Sarah M Tete, Sarah Lartey, Geir Bredholt & Rebecca J Cox * Department of Clinical Science, KG Jebsen Centre for
Influenza Vaccines, University of Bergen, Bergen, Norway Sarah M Tete, Sarah Lartey & Rebecca J Cox * Department of Research & Development, Haukeland University Hospital, Bergen,
Norway Sarah M Tete, Sarah Lartey & Rebecca J Cox * Department of Microbiology, Icahn School of Medicine at Mount Sinai, New York, NY, USA Florian Krammer * National Institute for
Biological Standards and Control, Hertfordshire, UK John Wood * Section for Infectious Diseases, Haukeland University Hospital, Bergen, Norway Steinar Skrede Authors * Sarah M Tete View
author publications You can also search for this author inPubMed Google Scholar * Florian Krammer View author publications You can also search for this author inPubMed Google Scholar * Sarah
Lartey View author publications You can also search for this author inPubMed Google Scholar * Geir Bredholt View author publications You can also search for this author inPubMed Google
Scholar * John Wood View author publications You can also search for this author inPubMed Google Scholar * Steinar Skrede View author publications You can also search for this author
inPubMed Google Scholar * Rebecca J Cox View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS S.M.T. performed the experiments, was involved in
interpreting the data and prepared the manuscript. F.K. provided the recombinant HA used in the experiments and was involved in analysis and interpreting the data. S.L. performed some of the
experiments and contributed to interpretation of the results. G.B contributed to experimental design and interpretation of results. J.W., S.S. and R.J.C. contributed to the study design,
protocol design and interpretation of the results. All authors critically reviewed the manuscript and approved the final article. S.M.T. and R.J.C. are the guarantors. CORRESPONDING AUTHORS
Correspondence to Sarah M Tete or Rebecca J Cox. ETHICS DECLARATIONS COMPETING INTERESTS F.K. received support from NIAID through U19 AI109946 and the Centers of Influenza Virus Research and
Surveillance (CEIRS) contract HHSN272201400008C. The remaining authors declare no conflict of interest. ADDITIONAL INFORMATION Supplementary Information accompanies the paper on the _npj
Vaccines_ website (http://www.nature.com/npjvaccines) SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE S1 (JPG 47 KB) RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons
Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the
credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of
this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Tete, S., Krammer, F., Lartey, S. _et al._ Dissecting the
hemagglutinin head and stalk-specific IgG antibody response in healthcare workers following pandemic H1N1 vaccination. _npj Vaccines_ 1, 16001 (2016).
https://doi.org/10.1038/npjvaccines.2016.1 Download citation * Received: 07 March 2016 * Revised: 10 May 2016 * Accepted: 18 May 2016 * Published: 28 July 2016 * DOI:
https://doi.org/10.1038/npjvaccines.2016.1 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative







