- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
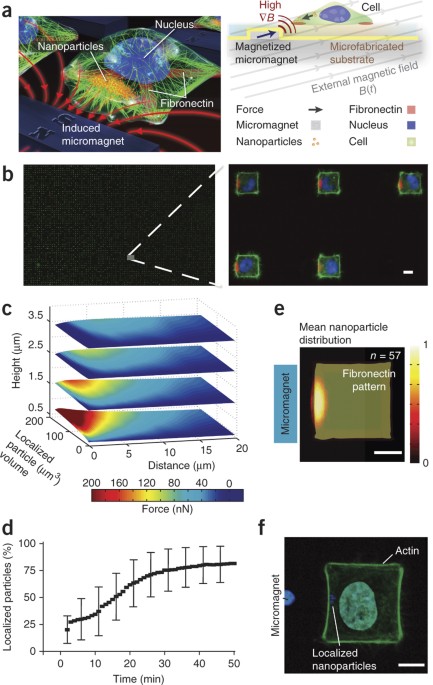
ABSTRACT We report a technique for generating controllable, time-varying and localizable forces on arrays of cells in a massively parallel fashion. To achieve this, we grow magnetic
nanoparticle–dosed cells in defined patterns on micromagnetic substrates. By manipulating and coalescing nanoparticles within cells, we apply localized nanoparticle-mediated forces
approaching cellular yield tensions on the cortex of HeLa cells. We observed highly coordinated responses in cellular behavior, including the p21-activated kinase–dependent generation of
active, leading edge–type filopodia and biasing of the metaphase plate during mitosis. The large sample size and rapid sample generation inherent to this approach allow the analysis of cells
at an unprecedented rate: in a single experiment, potentially tens of thousands of cells can be stimulated for high statistical accuracy in measurements. This technique shows promise as a
tool for both cell analysis and control. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through
your institution Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant
access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions *
Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS ACOUSTOELECTRONIC NANOTWEEZERS ENABLE DYNAMIC AND LARGE-SCALE CONTROL OF NANOMATERIALS Article Open access 22
June 2021 ACOUSTIC TWEEZERS FOR HIGH-THROUGHPUT SINGLE-CELL ANALYSIS Article 19 July 2023 EMERGING MECHANOBIOLOGY TECHNIQUES TO PROBE INTRACELLULAR MECHANICS Article Open access 04 April
2025 REFERENCES * Ingber, D.E. Tensegrity: the architectural basis of cellular mechanotransduction. _Annu. Rev. Physiol._ 59, 575–599 (1997). Article CAS Google Scholar * Orr, A.W.,
Helmke, B.P., Blackman, B.R. & Schwartz, M.A. Mechanisms of mechanotransduction. _Dev. Cell_ 10, 11–20 (2006). Article CAS Google Scholar * Chen, C.S. Mechanotransduction—a field
pulling together? _J. Cell Sci._ 121, 3285–3292 (2008). Article CAS Google Scholar * Henderson, E., Haydon, P.G. & Sakaguchi, D.S. Actin filament dynamics in living glial cells imaged
by atomic force microscopy. _Science_ 257, 1944–1946 (1992). Article CAS Google Scholar * Charras, G.T. & Horton, M.A. Single cell mechanotransduction and its modulation analyzed by
atomic force microscope indentation. _Biophys. J._ 82, 2970–2981 (2002). Article CAS Google Scholar * Prass, M., Jacobson, K., Mogilner, A. & Radmacher, M. Direct measurement of the
lamellipodial protrusive force in a migrating cell. _J. Cell Biol._ 174, 767–772 (2006). Article CAS Google Scholar * Dai, J. & Sheetz, M.P. Mechanical properties of neuronal growth
cone membranes studied by tether formation with laser optical tweezers. _Biophys. J._ 68, 988–996 (1995). Article CAS Google Scholar * Wang, N., Butler, J.P. & Ingber, D.E.
Mechanotransduction across the cell surface and through the cytoskeleton. _Science_ 260, 1124–1127 (1993). Article CAS Google Scholar * Laurent, V.M. et al. Assessment of mechanical
properties of adherent living cells by bead micromanipulation: comparison of magnetic twisting cytometry vs optical tweezers. _J. Biomech. Eng._ 124, 408–421 (2002). Article Google Scholar
* Huang, H. et al. Three-dimensional cellular deformation analysis with a two-photon magnetic manipulator workstation. _Biophys. J._ 82, 2211–2223 (2002). Article CAS Google Scholar *
Marcy, Y., Prost, J., Carlier, M.-F. & Sykes, C. Forces generated during actin-based propulsion: a direct measurement by micromanipulation. _Proc. Natl. Acad. Sci. USA_ 101, 5992–5997
(2004). Article CAS Google Scholar * Hochmuth, R.M. Micropipette aspiration of living cells. _J. Biomech._ 33, 15–22 (2000). Article CAS Google Scholar * Evans, E., Ritchie, K. &
Merkel, R. Sensitive force technique to probe molecular adhesion and structural linkages at biological interfaces. _Biophys. J._ 68, 2580–2587 (1995). Article CAS Google Scholar * Pelham,
R.J. Jr. & Wang, Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. _Proc. Natl. Acad. Sci. USA_ 94, 13661–13665 (1997). Article CAS Google Scholar *
Banes, A.J. et al. Mechanoreception at the cellular level: the detection, interpretation, and diversity of responses to mechanical signals. _Biochem. Cell Biol._ 73, 349–365 (1995). Article
CAS Google Scholar * Sniadecki, N.J. et al. Magnetic microposts as an approach to apply forces to living cells. _Proc. Natl. Acad. Sci. USA_ 104, 14553–14558 (2007). Article CAS Google
Scholar * Hui, E.E. & Bhatia, S.N. Micromechanical control of cell-cell interactions. _Proc. Natl. Acad. Sci. USA_ 104, 5722–5726 (2007). Article CAS Google Scholar * Tanase, M. et
al. Assembly of multicellular constructs and microarrays of cells using magnetic nanowires. _Lab Chip_ 5, 598–605 (2005). Article CAS Google Scholar * Fink, J. et al. External forces
control mitotic spindle positioning. _Nat. Cell Biol._ 13, 771–778 (2011). Article CAS Google Scholar * Gao, J. et al. Intracellular spatial control of fluorescent magnetic nanoparticles.
_J. Am. Chem. Soc._ 130, 3710–3711 (2008). Article CAS Google Scholar * de Vries, A.H., Krenn, B.E., van Driel, R. & Kanger, J. Micro magnetic tweezers for nanomanipulation inside
live cells. _Biophys. J._ 88, 2137–2144 (2005). Article CAS Google Scholar * Tseng, P., Di Carlo, D. & Judy, J.W. Rapid and dynamic intracellular patterning of cell-internalized
magnetic fluorescent nanoparticles. _Nano Lett._ 9, 3053–3059 (2009). Article CAS Google Scholar * Dobson, J. Remote control of cellular behaviour with magnetic nanoparticles. _Nat.
Nanotechnol._ 3, 139–143 (2008). Article CAS Google Scholar * Mannix, R.J. et al. Nanomagnetic actuation of receptor-mediated signal transduction. _Nat. Nanotechnol._ 3, 36–40 (2008).
Article CAS Google Scholar * Huang, H., Delikanli, S., Zeng, H., Ferkey, D.M. & Pralle, A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles.
_Nat. Nanotechnol._ 5, 602–606 (2010). Article CAS Google Scholar * Glickman, M. et al. High-performance lateral-actuating magnetic MEMS switch. _J. Microelectromech. Syst._ 20, 842–851
(2011). Article CAS Google Scholar * Pai, J.-H. Photoresist with low fluorescence for bioanalytical applications. _Anal. Chem._ 79, 8774–8780 (2007). Article CAS Google Scholar *
Schäffer, E., Nørrelykke, S.F. & Howard, J. Surface forces and drag coefficients of microspheres near a plane surface measured with optical tweezers. _Langmuir_ 23, 3654–3665 (2007).
Article Google Scholar * Abraham, V.C., Krishnamurthi, V., Taylor, D.L. & Lanni, F. The actin-based nanomachine at the leading edge of migrating cells. _Biophys. J._ 77, 1721–1732
(1999). Article CAS Google Scholar * Berg, J.S. & Cheney, R.E. Myosin-X is an unconventional myosin that undergoes intrafilopodial motility. _Nat. Cell Biol._ 4, 246–250 (2002).
Article CAS Google Scholar * Papakonstanti, E.A. & Stournaras, C. Association of PI-3 kinase with PAK1 leads to actin phosphorylation and cytoskeletal reorganization. _Mol. Biol.
Cell_ 13, 2946–2962 (2002). Article CAS Google Scholar * Dharmawardhane, S., Brownson, D., Lennartz, M. & Bokoch, G.M. Localization of p21-activated kinase 1 (PAK1) to pseudopodia,
membrane ruffles, and phagocytic cups in activated human neutrophils. _J. Leukoc. Biol._ 66, 521–527 (1999). Article CAS Google Scholar * Hahn, C. & Schwartz, M.A. Mechanotransduction
in vascular physiology and atherogenesis. _Nat. Rev. Mol. Cell Biol._ 10, 53–62 (2009). Article CAS Google Scholar * Tzima, E. et al. Activation of Rac1 by shear stress in endothelial
cells mediates both cytoskeletal reorganization and effects of gene expression. _EMBO J._ 21, 6791–6800 (2002). Article CAS Google Scholar * Zhang, H. et al. A tension-induced
mechanotransduction pathway promotes epithelial morphogenesis. _Nature_ 471, 99–103 (2011). Article CAS Google Scholar * Delorme-Walker, V.D. et al. Pak1 regulates focal adhesion
strength, myosin IIA distribution, and actin dynamics to optimize cell migration. _J. Cell Biol._ 193, 1289–1303 (2011). Article CAS Google Scholar * Van den Broeke, C. et al.
Alphaherpesvirus US3-mediated reorganization of the actin cytoskeleton is mediated by group A p21-activated kinases. _Proc. Natl. Acad. Sci. USA_ 106, 8707–8712 (2009). Article CAS Google
Scholar * Théry, M. et al. The extracellular matrix guides the orientation of the cell division axis. _Nat. Cell Biol._ 7, 947–953 (2005). Article Google Scholar * Théry, M.,
Jiménez-Dalmoroni, A., Racine, V., Bornens, M. & Jülicher, F. Experimental and theoretical study of mitotic spindle orientation. _Nature_ 447, 493–496 (2007). Article Google Scholar *
Pande, A.N., Kohler, R.H., Aikawa, E., Weissleder, R. & Jaffer, F.A. Detection of macrophage activity in atherosclerosis _in vivo_ using multichannel, high-resolution laser scanning
fluorescence microscopy. _J. Biomed. Opt._ 11, 021009 (2006). Article Google Scholar * Guillou, H. et al. Lamellipodia nucleation by filopodia depends on integrin occupancy and downstream
Rac1 signaling. _Exp. Cell Res._ 314, 478–488 (2008). Article CAS Google Scholar * Nolen, B.J. et al. Characterization of two classes of small molecule inhibitors of Arp2/3 complex.
_Nature_ 460, 1031–1034 (2009). Article CAS Google Scholar * Deacon, S.W. et al. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated
kinase. _Chem. Biol._ 15, 322–331 (2008). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was partially supported through the US National Institutes of Health
Director's New Innovator Award (1DP2OD007113). The authors thank M. Bachman and N. Gunn (University of California, Irvine) for samples of PSR; J. Harrison, M. Glickman and I. Goldberg
for assistance with the permalloy electroplating bath; members of the UCLA Advanced Light Microscopy Spectroscopy facility for assistance with confocal microscopy; K. Lin for high-speed
imaging assistance; I. Williams for running FACS; and engineers of the UCLA Nanolab for processing assistance. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Bioengineering,
University of California, Los Angeles (UCLA), Los Angeles, California, USA Peter Tseng, Jack W Judy & Dino Di Carlo * Department of Electrical Engineering, UCLA, Los Angeles, California,
USA Peter Tseng & Jack W Judy * California NanoSystems Institute, UCLA, Los Angeles, California, USA Jack W Judy & Dino Di Carlo * Jonsson Comprehensive Cancer Center, UCLA, Los
Angeles, California, USA Dino Di Carlo Authors * Peter Tseng View author publications You can also search for this author inPubMed Google Scholar * Jack W Judy View author publications You
can also search for this author inPubMed Google Scholar * Dino Di Carlo View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS P.T. and D.D.C.
contributed to the initial concept. P.T. and J.W.J. contributed to the fabrication design. D.D.C. and P.T. designed the integration of magnetic elements and single cells. P.T. developed the
final fabrication and cell-patterning protocols. P.T. fabricated the micromagnetic slides and conducted the cell experiments. J.W.J. and P.T. discussed the finite-element simulation. P.T.
designed the numerical analysis flow. P.T. and D.D.C. discussed and analyzed the numerical results. All authors wrote the manuscript. CORRESPONDING AUTHOR Correspondence to Dino Di Carlo.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TEXT AND FIGURES Supplementary Figures 1–9 (PDF 1355 kb)
MAGNETIC FLUORESCENT NANOPARTICLES COALESCE QUICKLY UNDER HIGH MAGNETIC FIELD STIMULATION (RESIN THICKNESS IS 0.5 ΜM). Nanoparticle assembly occurs over a period of ~30 min at the left edge
of the cell. As nanoparticles begin coalescing at the membrane edge, small clusters of nanoparticles enter temporary filopodial protrusions that extend beyond the edge. This effect
continues until around the 1-h mark, when the cell membrane begins to yield under the high tension until finally the nanoparticle cluster exhibits a 'pull-in' instability, and the
entire nanoparticle cluster protrudes from the edge of the cell membrane. Cell cytoplasm is labeled with calcein AM. (MOV 1472 kb) HIGH-SPEED IMAGES OF MAGNETIC BEADS MANIPULATED BY
INDIVIDUAL FERROMAGNETIC MICROMAGNETS. Video displays the trajectories of two magnetic beads moving along the substrate surface towards the magnetized elements using resins with thicknesses
of 2.5 and 5.3 μm, respectively. (AVI 970 kb) CONFOCAL MICROSCOPY _Z_ SLICES OF A SINGLE CELL UNDER MODERATE MAGNETIC NANOPARTICLE–MEDIATED TENSION. At the _z_ planes where the cell membrane
is under magnetic nanoparticle–induced tension, local effects are observed including (i) local deformation of the flanking stress fiber caused by the applied mechanical tension and (ii)
flanking actin-rich protrusions emanating from the regions of highest mechanical deformation. Positive myosin-X staining at the tips of protrusions indicate induced active, ECM-attachable
filopodia. (AVI 479 kb) CONFOCAL MICROSCOPY _Z_ SLICES SHOWING THE RICH BAND OF STRESS FIBER–LOCALIZED PHOSPHO-PAK PROGRESSING THROUGH THE CORTICAL REGIONS OF HIGH DEFORMATION. This
colocalization occurs whether or not the particular cell is expressing a high filopodial asymmetry and is distinct at regions directly above where the nanoparticles are localized. (AVI 1042
kb) CELL DIVIDING ALONG THE AXIS OF FORCE APPLICATION. The time-lapse video shows a single cell adhering to an I-shaped fibronectin pattern as it divides under high nanoparticle-induced
tension. As the cell undergoes and completes mitosis, the cell divides biased in the direction of the force generated by the magnetic nanoparticles. Upon successful division, both cells
adhere and move normally. The nanoparticles remain only in one of the daughter cells. (MOV 936 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Tseng,
P., Judy, J. & Di Carlo, D. Magnetic nanoparticle–mediated massively parallel mechanical modulation of single-cell behavior. _Nat Methods_ 9, 1113–1119 (2012).
https://doi.org/10.1038/nmeth.2210 Download citation * Received: 22 April 2012 * Accepted: 06 September 2012 * Published: 14 October 2012 * Issue Date: November 2012 * DOI:
https://doi.org/10.1038/nmeth.2210 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative





