- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
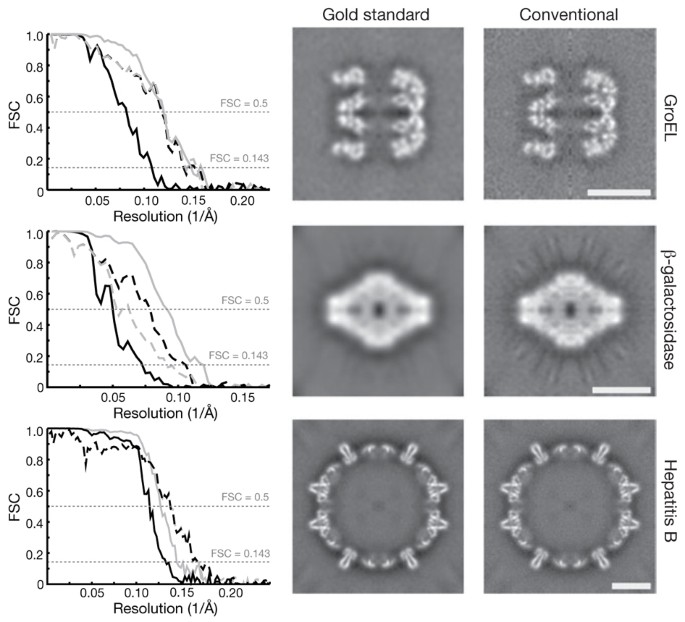
Access through your institution Buy or subscribe To the Editor: In the field of single-particle analysis of electron cryomicroscopy (cryo-EM) data, a growing concern that some resolution
claims might not be substantiated by the data has helped instigate community-wide efforts to develop new validation tools1. A known issue with commonly used cryo-EM structure determination
procedures is their tendency to overfit the data. Most procedures counter overfitting by low-pass filtering, but the effective frequencies for these filters are often based on suboptimal
Fourier shell correlation2 (FSC) procedures. In the suboptimal procedure, FSC curves are calculated between reconstructions from two halves of the data, and a single model is used to
determine the relative orientations of all particles. It is well known that bias toward noise in the single model may inflate the resulting resolution estimates. To illustrate this, we
applied the suboptimal procedure to a simulated cryo-EM data set of 20,212 GroEL particles. Whereas the reported resolution was 4.6 Å, the true resolution of the map was only 7.8 Å. Also,
the presence of expected density features in the map does not necessarily provide sufficient evidence for a resolution claim: we made convincing figures of apparent side-chain density that
in reality corresponded to overfitted noise (Supplementary Fig. 1). Consequently, overfitting may remain undetected, and interpretation of cryo-EM maps may be subject to errors. This is a
preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $259.00 per
year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated
during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support REFERENCES * Henderson, R. et al. _Structure_ 20,
205–214 (2012). Article CAS Google Scholar * Saxton, W.O. & Baumeister, W. _J. Microsc._ 127, 127–138 (1982). Article CAS Google Scholar * Stewart, A. & Grigorieff, N.
_Ultramicroscopy_ 102, 67–84 (2004). Article CAS Google Scholar * Scheres, S.H. _J. Mol. Biol._ 415, 406–418 (2012). Article CAS Google Scholar * Henderson, R. et al. _J. Mol. Biol._
413, 1028–1046 (2011). Article CAS Google Scholar * Scheres, S.H. et al. _Nat. Protoc._ 3, 977–990 (2008). Article CAS Google Scholar * Böttcher, B., Wynne, S.A. & Crowther, R.A.
_Nature_ 386, 88–91 (1997). Article Google Scholar * Rosenthal, P.B. & Henderson, R. _J. Mol. Biol._ 333, 721–745 (2003). Article CAS Google Scholar Download references
ACKNOWLEDGEMENTS We are grateful to T. Crowther and R. Henderson for helpful discussions and to J. Grimmett for help with computing. T. Crowther provided hepatitis B data, and the National
Center for Macromolecular Imaging, which is funded by US National Institutes of Health grant P41RR02250, provided GroEL data. This work was funded by the UK Medical Research Council through
grant MC_UP_A025_1013 to S.H.W.S. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Medical Research Council Laboratory of Molecular Biology, Cambridge, UK Sjors H W Scheres & Shaoxia Chen
Authors * Sjors H W Scheres View author publications You can also search for this author inPubMed Google Scholar * Shaoxia Chen View author publications You can also search for this author
inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Sjors H W Scheres. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY
INFORMATION SUPPLEMENTARY TEXT AND FIGURES Supplementary Figures 1–4, Supplementary Table 1, Supplementary Methods and Supplementary Software (PDF 765 kb) RIGHTS AND PERMISSIONS Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Scheres, S., Chen, S. Prevention of overfitting in cryo-EM structure determination. _Nat Methods_ 9, 853–854 (2012).
https://doi.org/10.1038/nmeth.2115 Download citation * Published: 29 July 2012 * Issue Date: September 2012 * DOI: https://doi.org/10.1038/nmeth.2115 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative
:max_bytes(150000):strip_icc():focal(216x0:218x2)/benedict-cumberbatch-1-435-4-20cc736017b24435a3498a49d7c22b0e.jpg)





