- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
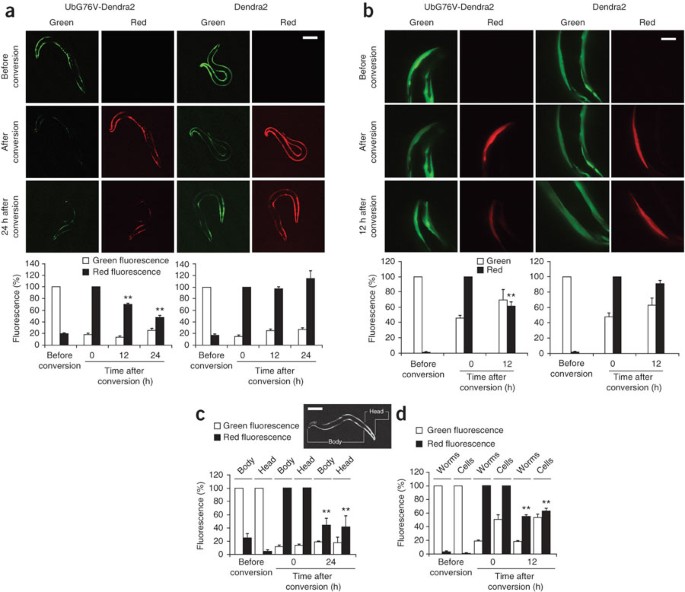
ABSTRACT The ubiquitin-proteasome system (UPS) orchestrates many cellular and tissue-specific processes by degrading damaged and key regulatory proteins. To enable investigation of UPS
activity in different cell types in a living animal, we developed a photoconvertible fluorescent UPS reporter system for live imaging and quantification of protein degradation in
_Caenorhabditis elegans_. Our reporter consists of the photoconvertible fluorescent protein Dendra2 targeted for proteasomal degradation by fusion to the UbG76V mutant form of ubiquitin. In
contrast to previous reporters, this system permits quantification of UPS activity independently of protein synthesis. Our reporter revealed that UPS-mediated protein degradation varies in a
cell type–specific and age-dependent manner in _C. elegans_. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS
OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on
SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about
institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS TAGGING THE PROTEASOME ACTIVE SITE Β5 CAUSES TAG SPECIFIC PHENOTYPES IN YEAST
Article Open access 22 October 2020 REWIRING OF THE UBIQUITINATED PROTEOME DETERMINES AGEING IN _C. ELEGANS_ Article Open access 28 July 2021 PROFILING UBIQUITIN SIGNALLING WITH UBIMAX
REVEALS DNA DAMAGE- AND SCFΒ-TRCP1-DEPENDENT UBIQUITYLATION OF THE ACTIN-ORGANIZING PROTEIN DBN1 Article Open access 14 December 2023 CHANGE HISTORY * _ 17 JUNE 2010 In the version of this
paper originally published, a reference to previous work on the use of Dendra2 as a reporter for protein stability in cultured cells should have been included (ref. 35). The error has been
corrected in the PDF and HTML versions of the article. _ REFERENCES * Hanna, J. & Finley, D. A proteasome for all occasions. _FEBS Lett._ 581, 2854–2861 (2007). Article CAS PubMed
PubMed Central Google Scholar * Paul, S. Dysfunction of the ubiquitin-proteasome system in multiple disease conditions: therapeutic approaches. _Bioessays_ 30, 1172–1184 (2008). Article
CAS PubMed Google Scholar * Vernace, V.A., Schmidt-Glenewinkel, T. & Figueiredo-Pereira, M.E. Aging and regulated protein degradation: who has the UPPer hand? _Aging Cell_ 6, 599–606
(2007). Article CAS PubMed Google Scholar * Dantuma, N.P., Lindsten, K., Glas, R., Jellne, M. & Masucci, M.G. Short-lived green fluorescent proteins for quantifying
ubiquitin/proteasome-dependent proteolysis in living cells. _Nat. Biotechnol._ 18, 538–543 (2000). Article CAS PubMed Google Scholar * Bence, N.F., Sampat, R.M. & Kopito, R.R.
Impairment of the ubiquitin-proteasome system by protein aggregation. _Science_ 292, 1552–1555 (2001). Article CAS PubMed Google Scholar * Holmberg, C.I., Staniszewski, K.E., Mensah,
K.N., Matouschek, A. & Morimoto, R.I. Inefficient degradation of truncated polyglutamine proteins by the proteasome. _EMBO J._ 23, 4307–4318 (2004). Article CAS PubMed PubMed Central
Google Scholar * Lindsten, K., Menéndez-Benito, V., Masucci, M.G. & Dantuma, N.P. A transgenic mouse model of the ubiquitin/proteasome system. _Nat. Biotechnol._ 21, 897–902 (2003).
Article CAS PubMed Google Scholar * Kumarapeli, A.R. et al. A novel transgenic mouse model reveals deregulation of the ubiquitin-proteasome system in the heart by doxorubicin. _FASEB J._
19, 2051–2053 (2005). Article PubMed Google Scholar * Bove, J. et al. Proteasome inhibition and Parkinson′s disease modeling. _Ann. Neurol._ 60, 260–264 (2006). Article CAS PubMed
Google Scholar * Cook, C. et al. Aging is not associated with proteasome impairment in UPS reporter mice. _PLoS One_ 4, e5888 (2009). Article PubMed PubMed Central Google Scholar *
Alvarez-Castelao, B., Martin-Guerrero, I., Garcia-Orad, A. & Castano, J.G. CMV promoter up-regulation is the major cause of increased protein levels of unstable reporter proteins after
treatment of living cells with proteasome inhibitors. _J. Biol. Chem._ 41, 28253–28262 (2009). Article Google Scholar * Johnson, E.S., Bartel, B., Seufert, W. & Varshavsky, A.
Ubiquitin as a degradation signal. _EMBO J._ 11, 497–505 (1992). Article CAS PubMed PubMed Central Google Scholar * Chudakov, D.M., Lukyanov, S. & Lukyanov, K.A. Tracking
intracellular protein movements using photoswitchable fluorescent proteins PS-CFP2 and Dendra2. _Nat. Protocols_ 2, 2024–2032 (2007). Article CAS PubMed Google Scholar * Jantsch-Plunger,
V. & Fire, A. Combinatorial structure of a body muscle-specific transcriptional enhancer in _Caenorhabditis elegans_. _J. Biol. Chem._ 269, 27021–27028 (1994). CAS PubMed Google
Scholar * Shimada, M., Kanematsu, K., Tanaka, K., Yokosawa, H. & Kawahara, H. Proteasomal ubiquitin receptor RPN-10 controls sex determination in _Caenorhabditis elegans_. _Mol. Biol.
Cell_ 17, 5356–5371 (2006). Article CAS PubMed PubMed Central Google Scholar * van Nocker, S. et al. The multiubiquitin-chain-binding protein Mcb1 is a component of the 26S proteasome
in _Saccharomyces cerevisiae_ and plays a nonessential, substrate-specific role in protein turnover. _Mol. Cell. Biol._ 16, 6020–6028 (1996). Article CAS PubMed PubMed Central Google
Scholar * Stack, J.H., Whitney, M., Rodems, S.M. & Pollok, B.A. A ubiquitin-based tagging system for controlled modulation of protein stability. _Nat. Biotechnol._ 18, 1298–1302 (2000).
Article CAS PubMed Google Scholar * Verhoef, L.G. et al. Minimal length requirement for proteasomal degradation of ubiquitin-dependent substrates. _FASEB J._ 1, 123–133 (2008). Google
Scholar * Kim, H.T., Kim, K.P., Uchiki, T., Gygi, S.P. & Goldberg, A.L. S5a promotes protein degradation by blocking synthesis of nondegradable forked ubiquitin chains. _EMBO J._ 13,
1867–1877 (2009). Article Google Scholar * Breusing, N. & Grune, T. Regulation of proteasome-mediated protein degradation during oxidative stress and aging. _Biol. Chem._ 389, 203–209
(2008). Article CAS PubMed Google Scholar * Yun, C. et al. Proteasomal adaptation to environmental stress links resistance to proteotoxicity with longevity in _Caenorhabditis elegans_.
_Proc. Natl. Acad. Sci. USA_ 105, 7094–7099 (2008). Article CAS PubMed PubMed Central Google Scholar * Ghazi, A., Henis-Korenblit, S. & Kenyon, C. Regulation of _Caenorhabditis
elegans_ lifespan by a proteasomal E3 ligase complex. _Proc. Natl. Acad. Sci. USA_ 104, 5947–5952 (2007). Article CAS PubMed PubMed Central Google Scholar * Walker, G.A. & Lithgow,
G.J. Lifespan extension in _C. elegans_ by a molecular chaperone dependent upon insulin-like signals. _Aging Cell_ 2, 131–139 (2003). Article CAS PubMed Google Scholar * Yokoyama, K. et
al. Extended longevity of _Caenorhabditis elegans_ by knocking in extra copies of hsp70F, a homolog of mot-2 (mortalin)/mthsp70/Grp75. _FEBS Lett._ 516, 53–57 (2002). Article CAS PubMed
Google Scholar * Hsu, A.L., Murphy, C.T. & Kenyon, C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. _Science_ 300, 1142–1145 (2003). Article CAS PubMed
Google Scholar * Mehta, R. et al. Proteasomal regulation of the hypoxic response modulates aging in _C. elegans_. _Science_ 324, 1196–1198 (2009). Article CAS PubMed PubMed Central
Google Scholar * Li, W., Gao, B., Lee, S.M., Bennett, K. & Fang, D. RLE-1, an E3 ubiquitin ligase, regulates _C. elegans_ aging by catalyzing DAF-16 polyubiquitination. _Dev. Cell_ 12,
235–246 (2007). Article CAS PubMed Google Scholar * Morley, J.F. & Morimoto, R.I. Regulation of longevity in _Caenorhabditis elegans_ by heat shock factor and molecular chaperones.
_Mol. Biol. Cell_ 15, 657–664 (2004). Article CAS PubMed PubMed Central Google Scholar * Herndon, L.A. et al. Stochastic and genetic factors influence tissue-specific decline in ageing
_C. elegans_. _Nature_ 419, 808–814 (2002). Article CAS PubMed Google Scholar * Brenner, S. The genetics of _Caenorhabditis elegans_. _Genetics_ 77, 71–94 (1974). CAS PubMed PubMed
Central Google Scholar * Brignull, H.R., Moore, F.E., Tang, S.J. & Morimoto, R.I. Polyglutamine proteins at the pathogenic threshold display neuron-specific aggregation in a
pan-neuronal _Caenorhabditis elegans_ model. _J. Neurosci._ 26, 7597–7606 (2006). Article CAS PubMed PubMed Central Google Scholar * Mello, C.C., Kramer, J.M., Stinchcomb, D. &
Ambros, V. Efficient gene transfer in _C. elegans_: extrachromosomal maintenance and integration of transforming sequences. _EMBO J._ 10, 3959–3970 (1991). Article CAS PubMed PubMed
Central Google Scholar * Timmons, L., Court, D.L. & Fire, A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in _Caenorhabditis elegans_.
_Gene_ 263, 103–112 (2001). Article CAS PubMed Google Scholar * Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. _Nature_ 227, 680–685
(1970). Article CAS PubMed Google Scholar * Zhang, L. et al. Method for real-time monitoring of protein degradation at the single cell level. _Biotechniques_ 42, 446–450 (2007). Article
CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS This study was supported by grants to C.I.H. from the Academy of Finland (113485 and 118450), the International Human
Frontier Science Program Organization, Biocentrum Helsinki, the Sigrid Jusélius Foundation and the Magnus Ehrnrooth Foundation. G.H. was additionally supported by the University of Helsinki
and O.M. by the Helsinki Biomedical Graduate School. We thank members of the Caenorhabditis Genetics Center for providing the N2 wild-type worms, S. Mitani (National Bioresource Project for
the Nematode, Japan) for the _rpn-10_ mutant worms, R.I. Morimoto (Northwestern University) for the _PF25B3.3::yfp_ expression vector and members of the Biomedicum Helsinki Molecular Imaging
Unit for their help with confocal microscopy and imaging. AUTHOR INFORMATION Author notes * Geert Hamer and Olli Matilainen: These authors contributed equally to this work. AUTHORS AND
AFFILIATIONS * Molecular Cancer Biology Program and Institute for Biomedicine, Biomedicum Helsinki, University of Helsinki, Helsinki, Finland Geert Hamer, Olli Matilainen & Carina I
Holmberg Authors * Geert Hamer View author publications You can also search for this author inPubMed Google Scholar * Olli Matilainen View author publications You can also search for this
author inPubMed Google Scholar * Carina I Holmberg View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS G.H. and O.M. developed the method,
performed the experiments, analyzed the data, made the figures and wrote the paper. C.I.H. developed the method, analyzed the data, wrote the paper and supported the project. CORRESPONDING
AUTHOR Correspondence to Carina I Holmberg. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TEXT AND
FIGURES Supplementary Figures 1–9 and Supplementary Tables 1–2 (PDF 2945 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hamer, G., Matilainen, O.
& Holmberg, C. A photoconvertible reporter of the ubiquitin-proteasome system _in vivo_. _Nat Methods_ 7, 473–478 (2010). https://doi.org/10.1038/nmeth.1460 Download citation * Received:
25 January 2010 * Accepted: 08 April 2010 * Published: 09 May 2010 * Issue Date: June 2010 * DOI: https://doi.org/10.1038/nmeth.1460 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative






:max_bytes(150000):strip_icc():focal(319x0:321x2)/people_social_image-60e0c8af9eb14624a5b55f2c29dbe25b.png)