- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
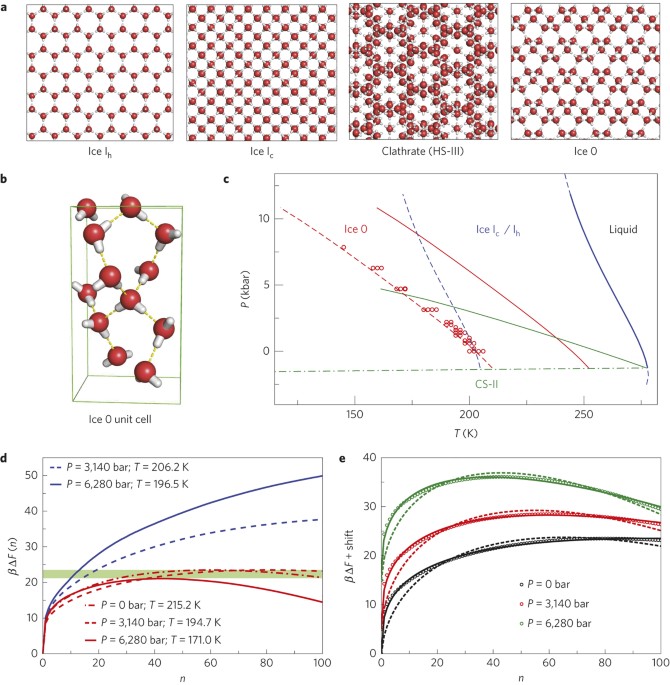
The homogeneous crystallization of water at low temperature is believed to occur through the direct nucleation of cubic (Ic) and hexagonal (Ih) ices. Here, we provide evidence from molecular
simulations that the nucleation of ice proceeds through the formation of a new metastable phase, which we name Ice 0. We find that Ice 0 is structurally similar to the supercooled liquid,
and that on growth it gradually converts into a stacking of Ice Ic and Ih. We suggest that this mechanism provides a thermodynamic explanation for the location and pressure dependence of the
homogeneous nucleation temperature, and that Ice 0 controls the homogeneous nucleation of low-pressure ices, acting as a precursor to crystallization in accordance with Ostwald’s step rule
of phases. Our findings show that metastable crystalline phases of water may play roles that have been largely overlooked.
We are grateful to F. Sciortino for a critical reading of the manuscript and to J. Doye and A. Reinhardt for useful discussions. This study was partly supported by Grants-in-Aid for
Scientific Research (S) and Specially Promoted Research from the Japan Society for the Promotion of Science (JSPS), the Aihara Project, the FIRST programme from JSPS, initiated by the
Council for Science and Technology Policy (CSTP), a JSPS short-term fellowship for F.R., and a JSPS Postdoctoral Fellowship for J.R.
John Russo and Flavio Romano: These authors contributed equally to this work.
Institute of Industrial Science, University of Tokyo, 4-6-1 Komaba, Meguro-ku Tokyo 153-8505, Japan,
Department of Chemistry, Physical and Theoretical Chemistry Laboratory, University of Oxford, South Parks Road Oxford OX1 3QZ, UK,
J.R. and F.R. performed the numerical simulations and the data analysis. H.T. proposed and supervised the study. All authors discussed the results and contributed to the writing of the
manuscript.
Anyone you share the following link with will be able to read this content:







