- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Cage structures exhibit inherent high symmetry and beauty, and both naturally occurring and synthetic molecular-scale cages have been discovered. Their characteristic high surface
area and voids have led to their use as catalysts and catalyst supports, filtration media and gas storage materials1,2. Nanometre-scale cage structures have also been synthesized, notably
noble-metal cube-shaped cages prepared by galvanic displacement with promising applications in drug delivery and catalysis3,4,5,6. Further functionality for nanostructures in general is
provided by the concept of hybrid nanoparticles combining two disparate materials on the same system to achieve synergistic properties stemming from unusual material combinations7,8,9,10,11.
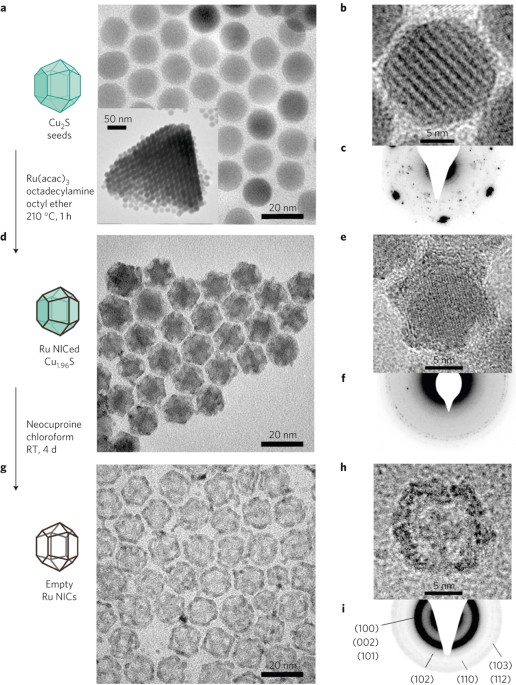
We report the integration of the two powerful concepts of cages and hybrid nanoparticles. A previously unknown edge growth mechanism has led to a new type of cage-structured hybrid
metal–semiconductor nanoparticle; a ruthenium cage was grown selectively on the edges of a faceted copper(I) sulphide nanocrystal, contrary to the more commonly observed facet and island
growth modes of other hybrids7,12,13,14,15. The cage motif was extended by exploiting the open frame to achieve empty cages and cages containing other semiconductors. Such previously unknown
nano-inorganic cage structures with variable cores and metal frames manifest new chemical, optical and electronic properties and demonstrate possibilities for uses in electrocatalysis.
Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this
journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now
Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer
support SIMILAR CONTENT BEING VIEWED BY OTHERS EPITAXIAL GROWTH OF METAL-ORGANIC FRAMEWORK NANOSHEETS INTO SINGLE-CRYSTALLINE ORTHOGONAL ARRAYS Article Open access 18 September 2023 THE
MULTIFACETED ROLES OF M_N_L2_N_ CAGES IN CATALYSIS Article 24 July 2024 MONOMICELLAR ASSEMBLY TO SYNTHESIZE STRUCTURED AND FUNCTIONAL MESOPOROUS CARBONACEOUS NANOMATERIALS Article 14
December 2022 REFERENCES * Eddaoudi, M. et al. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. _Science_ 295, 469–472 (2002).
Article CAS Google Scholar * Davis, M. E. Ordered porous materials for emerging applications. _Nature_ 417, 813–821 (2002). Article CAS Google Scholar * Skrabalak, S. E., Au, L., Li,
X. D. & Xia, Y. Facile synthesis of Ag nanocubes and Au nanocages. _Nature Protoc._ 2, 2182–2190 (2007). Article CAS Google Scholar * Skrabalak, S. E. et al. Gold nanocages:
Synthesis, properties, and applications. _Acc. Chem. Res._ 41, 1587–1595 (2008). Article CAS Google Scholar * Yavuz, M. S. et al. Gold nanocages covered by smart polymers for controlled
release with near-infrared light. _Nature Mater._ 8, 935–939 (2009). Article CAS Google Scholar * Zeng, J., Zhang, Q., Chen, J. Y. & Xia, Y. N. A comparison study of the catalytic
properties of Au-based nanocages, nanoboxes, and nanoparticles. _Nano Lett._ 10, 30–35 (2010). Article CAS Google Scholar * Costi, R., Saunders, A. E. & Banin, U. Colloidal hybrid
nanostructures: A new type of functional materials. _Angew. Chem. Int. Ed._ 49, 4878–4897 (2010). Article CAS Google Scholar * Mokari, T., Rothenberg, E., Popov, I., Costi, R. &
Banin, U. Selective growth of metal tips onto semiconductor quantum rods and tetrapods. _Science_ 304, 1787–1790 (2004). Article CAS Google Scholar * Cozzoli, P. D., Pellegrino, T. &
Manna, L. Synthesis, properties and perspectives of hybrid nanocrystal structures. _Chem. Soc. Rev._ 35, 1195–1208 (2006). Article CAS Google Scholar * Wetz, F. et al. Hybrid Co–Au
nanorods: Controlling Au nucleation and location. _Angew. Chem. Int. Ed._ 46, 7079–7081 (2007). Article CAS Google Scholar * Habas, S. E., Yang, P. D. & Mokari, T. Selective growth of
metal and binary metal tips on CdS nanorods. _J. Am. Chem. Soc._ 130, 3294–3295 (2008). Article CAS Google Scholar * Sadtler, B. et al. Selective facet reactivity during cation exchange
in cadmium sulfide nanorods. _J. Am. Chem. Soc._ 131, 5285–5293 (2009). Article CAS Google Scholar * Han, W. et al. Synthesis and shape-tailoring of copper sulfide/indium sulfide-based
nanocrystals. _J. Am. Chem. Soc._ 130, 13152–13161 (2008). Article CAS Google Scholar * Shi, W. L. et al. A general approach to binary and ternary hybrid nanocrystals. _Nano Lett._ 6,
875–881 (2006). Article CAS Google Scholar * Menagen, G., Macdonald, J. E., Shemesh, Y., Popov, I. & Banin, U. Au growth on semiconductor nanorods: Photoinduced versus thermal growth
mechanisms. _J. Am. Chem. Soc._ 131, 17406–17411 (2009). Article CAS Google Scholar * Figuerola, A. et al. End-to-end assembly of shape-controlled nanocrystals via a nanowelding approach
mediated by gold domains. _Adv. Mater._ 21, 550–554 (2009). Article CAS Google Scholar * Maynadié, J. et al. Cobalt growth on the tips of CdSe nanorods. _Angew. Chem. Int. Ed._ 48,
1814–1817 (2009). Article Google Scholar * Zhao, N., Liu, K., Greener, J., Nie, Z. H. & Kumacheva, E. Close-packed superlattices of side-by-side assembled Au–CdSe nanorods. _Nano
Lett._ 9, 3077–3081 (2009). Article CAS Google Scholar * Choi, S. H. et al. Simple and generalized synthesis of semiconducting metal sulfide nanocrystals. _Adv. Funct. Mater._ 19,
1645–1649 (2009). Article CAS Google Scholar * Joint Committee on Powder Diffraction Standards (JSPDS) cards employed for structural determination: hcp ruthenium: 03-065-1863, low
chalcocite: 03-033-0490, djurleite: 00-034-0660, hexagonal CdS: 01-077-2306, PbS: 03-065-2935. * Zhao, F. H. et al. Controlled growth of Cu2S hexagonal microdisks and their optical
properties. _J. Phys. Chem. Solids_ 67, 1786–1791 (2006). Article CAS Google Scholar * Midgley, P. A. & Dunin-Borkowski, R. E. Electron tomography and holography in materials science.
_Nature Mater._ 8, 271–280 (2009). Article CAS Google Scholar * Bar Sadan, M., Wolf, S. G. & Houben, L. Bright-field electron tomography of individual inorganic fullerene-like
structures. _Nanoscale_ 2, 423–428 (2010). Article CAS Google Scholar * Zhao, Y. X. et al. Plasmonic Cu2−_x_S nanocrystals: Optical and structural properties of copper-deficient copper(I)
sulfides. _J. Am. Chem. Soc._ 131, 4253–4261 (2009). Article CAS Google Scholar * Wu, Y., Wadia, C., Ma, W. L., Sadtler, B. & Alivisatos, A. P. Synthesis and photovoltaic application
of copper(I) sulfide nanocrystals. _Nano Lett._ 8, 2551–2555 (2008). Article CAS Google Scholar * Talapin, D. V., Yu, H., Shevchenko, E. V., Lobo, A. & Murray, C. B. Synthesis of
colloidal PbSe/PbS core–shell nanowires and PbS/Au nanowire-nanocrystal heterostructures. _J. Phys. Chem. C_ 111, 14049–14054 (2007). Article CAS Google Scholar * Mokari, T., Sztrum, C.
G., Salant, A., Rabani, E. & Banin, U. Formation of asymmetric one-sided metal-tipped semiconductor nanocrystal dots and rods. _Nature Mater._ 4, 855–863 (2005). Article CAS Google
Scholar * Myung, Y. et al. Nonenzymatic amperometric glucose sensing of platinum, copper sulfide, and tin oxide nanoparticle-carbon nanotube hybrid nanostructures. _J. Phys. Chem. C_ 113,
1251–1259 (2009). Article CAS Google Scholar * Luther, J. M., Zheng, H. M., Sadtler, B. & Alivisatos, A. P. Synthesis of PbS nanorods and other ionic nanocrystals of complex
morphology by sequential cation exchange reactions. _J. Am. Chem. Soc._ 131, 16851–16857 (2009). Article CAS Google Scholar * Connor, S. T., Hsu, C. M., Weil, B. D., Aloni, S. & Cui,
Y. Phase transformation of biphasic Cu2S-CuInS2 to monophasic CuInS2 nanorods. _J. Am. Chem. Soc._ 131, 4962–4966 (2009). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS
Partial financial support by the Israel Science Foundation (grant 972/08), and the ERC grant DCENSY is acknowledged. U.B. thanks the Alfred and Erica Larisch Memorial Chair in Solar Energy.
M.B.S. thanks the Minerva Fellowship program funded by the German Federal Ministry for Education and Research and the Sara Lee Schupf Postdoctoral Fellowship. The authors also thank D.
Mandler for use of electrochemisty instrumentation. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute of Chemistry, The Hebrew University of Jerusalem, Jerusalem 91904, Israel Janet E.
Macdonald & Uri Banin * The Center for Nanoscience and Nanotechnology, The Hebrew University of Jerusalem, Jerusalem 91904, Israel Janet E. Macdonald, Inna Popov & Uri Banin * Ernst
Ruska-Centre for Microscopy and Spectroscopy with Electrons, Research Centre Juelich, 52425 Juelich, Germany Maya Bar Sadan & Lothar Houben Authors * Janet E. Macdonald View author
publications You can also search for this author inPubMed Google Scholar * Maya Bar Sadan View author publications You can also search for this author inPubMed Google Scholar * Lothar Houben
View author publications You can also search for this author inPubMed Google Scholar * Inna Popov View author publications You can also search for this author inPubMed Google Scholar * Uri
Banin View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.E.M. and U.B. designed the experiments and wrote the manuscript. J.E.M. carried out
the experiments, materials characterization and analysis. I.P. assisted with HAADF-STEM and energy-dispersive X-ray spectroscopy measurements and provided commentary on the manuscript and
materials analysis. M.B.S. carried out the tomography experiments and the analysis of its data and wrote parts of the manuscripts. L.H. wrote the tomographic processing software and assisted
in the reconstruction, provided the aberration-corrected HAADF-STEM images and commented on the manuscript. CORRESPONDING AUTHOR Correspondence to Uri Banin. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION (PDF 479 KB) SUPPLEMENTARY INFORMATION Supplementary Movie 1 (MOV 1567 kb)
SUPPLEMENTARY INFORMATION Supplementary Movie 2 (MOV 2992 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Macdonald, J., Bar Sadan, M., Houben, L.
_et al._ Hybrid nanoscale inorganic cages. _Nature Mater_ 9, 810–815 (2010). https://doi.org/10.1038/nmat2848 Download citation * Received: 07 April 2010 * Accepted: 02 August 2010 *
Published: 19 September 2010 * Issue Date: October 2010 * DOI: https://doi.org/10.1038/nmat2848 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content:
Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative





