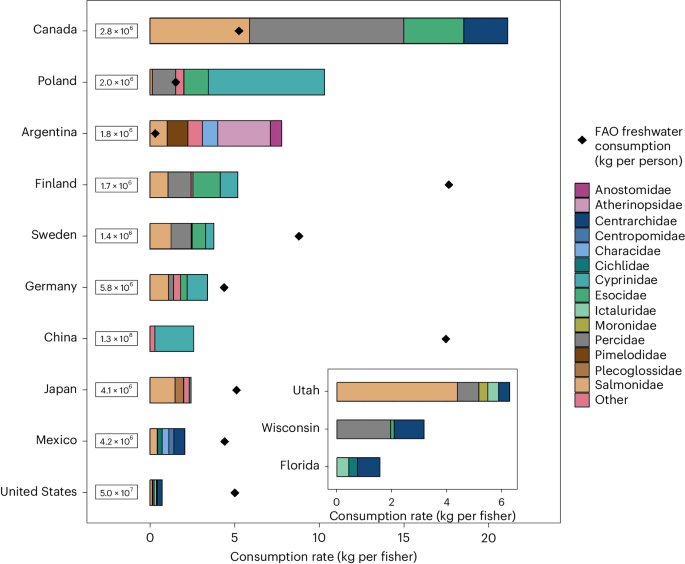
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT About 3,000 individuals in the United States are awaiting a donor heart; worldwide, 22 million individuals are living with heart failure. A bioartificial heart is a theoretical
alternative to transplantation or mechanical left ventricular support. Generating a bioartificial heart requires engineering of cardiac architecture, appropriate cellular constituents and
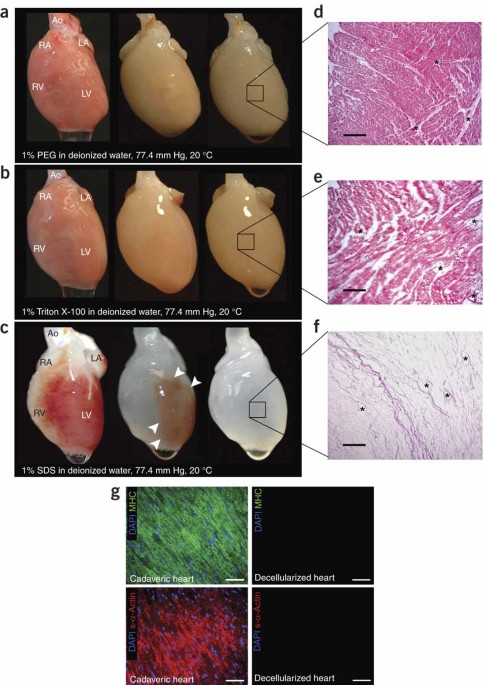
pump function. We decellularized hearts by coronary perfusion with detergents, preserved the underlying extracellular matrix, and produced an acellular, perfusable vascular architecture,
competent acellular valves and intact chamber geometry. To mimic cardiac cell composition, we reseeded these constructs with cardiac or endothelial cells. To establish function, we
maintained eight constructs for up to 28 d by coronary perfusion in a bioreactor that simulated cardiac physiology. By day 4, we observed macroscopic contractions. By day 8, under
physiological load and electrical stimulation, constructs could generate pump function (equivalent to about 2% of adult or 25% of 16-week fetal heart function) in a modified working heart
preparation. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe
to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF
Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact
customer support SIMILAR CONTENT BEING VIEWED BY OTHERS VERSATILE HUMAN CARDIAC TISSUES ENGINEERED WITH PERFUSABLE HEART EXTRACELLULAR MICROENVIRONMENT FOR BIOMEDICAL APPLICATIONS Article
Open access 22 March 2024 CHALLENGES AND OPPORTUNITIES FOR THE NEXT GENERATION OF CARDIOVASCULAR TISSUE ENGINEERING Article 05 September 2022 INTERINDIVIDUAL HETEROGENEITY AFFECTS THE
OUTCOME OF HUMAN CARDIAC TISSUE DECELLULARIZATION Article Open access 21 October 2021 REFERENCES * Kobashigawa, J.A. & Patel, J.K. Immunosuppression for heart transplantation: where are
we now? _Nat. Clin. Pract. Cardiovasc. Med._ 3, 203–212 (2006). Article CAS PubMed Google Scholar * Eschenhagen, T. & Zimmermann, W.H. Engineering myocardial tissue. _Circ. Res._ 97,
1220–1231 (2005). Article CAS PubMed Google Scholar * Zimmermann, W.H. et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. _Nat. Med._
12, 452–458 (2006). Article CAS PubMed Google Scholar * Sekine, H., Shimizu, T., Kosaka, S., Kobayashi, E. & Okano, T. Cardiomyocyte bridging between hearts and bioengineered
myocardial tissues with mesenchymal transition of mesothelial cells. _J. Heart Lung Transplant._ 25, 324–332 (2006). Article PubMed Google Scholar * Robinson, K.A. et al. Extracellular
matrix scaffold for cardiac repair. _Circulation_ 112, I135–I143 (2005). Article PubMed Google Scholar * Radisic, M., Deen, W., Langer, R. & Vunjak-Novakovic, G. Mathematical model of
oxygen distribution in engineered cardiac tissue with parallel channel array perfused with culture medium containing oxygen carriers. _Am. J. Physiol. Heart Circ. Physiol._ 288, H1278–H1289
(2005). Article CAS PubMed Google Scholar * Furuta, A. et al. Pulsatile cardiac tissue grafts using a novel three-dimensional cell sheet manipulation technique functionally integrates
with the host heart, _in vivo_. _Circ. Res._ 98, 705–712 (2006). Article CAS PubMed Google Scholar * Miyagawa, S. et al. Tissue cardiomyoplasty using bioengineered contractile
cardiomyocyte sheets to repair damaged myocardium: their integration with recipient myocardium. _Transplantation_ 80, 1586–1595 (2005). Article CAS PubMed Google Scholar * Park, H.,
Radisic, M., Lim, J.O., Chang, B.H. & Vunjak-Novakovic, G. A novel composite scaffold for cardiac tissue engineering. _In Vitro Cell. Dev. Biol. Anim._ 41, 188–196 (2005). Article CAS
PubMed Google Scholar * Dellgren, G., Eriksson, M.J., Brodin, L.A. & Radegran, K. Eleven years' experience with the Biocor stentless aortic bioprosthesis: clinical and hemodynamic
follow-up with long-term relative survival rate. _Eur. J. Cardiothorac. Surg._ 22, 912–921 (2002). Article PubMed Google Scholar * Rieder, E. et al. Decellularization protocols of
porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. _J. Thorac. Cardiovasc. Surg._ 127,
399–405 (2004). Article PubMed Google Scholar * Ketchedjian, A. et al. Recellularization of decellularized allograft scaffolds in ovine great vessel reconstructions. _Ann. Thorac. Surg._
79, 888–896 (2005). Article PubMed Google Scholar * Chen, R.N., Ho, H.O., Tsai, Y.T. & Sheu, M.T. Process development of an acellular dermal matrix (ADM) for biomedical applications.
_Biomaterials_ 25, 2679–2686 (2004). Article CAS PubMed Google Scholar * Gilbert, T.W., Sellaro, T.L. & Badylak, S.F. Decellularization of tissues and organs. _Biomaterials_ 27,
3675–3683 (2006). CAS PubMed Google Scholar * Gerecht-Nir, S. et al. Biophysical regulation during cardiac development and application to tissue engineering. _Int. J. Dev. Biol._ 50,
233–243 (2006). Article PubMed Google Scholar * Ossipow, V., Laemmli, U.K. & Schibler, U. A simple method to renature DNA-binding proteins separated by SDS-polyacrylamide gel
electrophoresis. _Nucleic Acids Res._ 21, 6040–6041 (1993). Article CAS PubMed PubMed Central Google Scholar * Paszek, M.J. et al. Tensional homeostasis and the malignant phenotype.
_Cancer Cell_ 8, 241–254 (2005). Article CAS PubMed Google Scholar * Johnson, P., Maxwell, D.J., Tynan, M.J. & Allan, L.D. Intracardiac pressures in the human fetus. _Heart_ 84,
59–63 (2000). Article CAS PubMed PubMed Central Google Scholar * Roy, S., Silacci, P. & Stergiopulos, N. Biomechanical properties of decellularized porcine common carotid arteries.
_Am. J. Physiol. Heart Circ. Physiol._ 289, H1567–H1576 (2005). Article CAS PubMed Google Scholar * Courtman, D.W. et al. Development of a pericardial acellular matrix biomaterial:
biochemical and mechanical effects of cell extraction. _J. Biomed. Mater. Res._ 28, 655–666 (1994). Article CAS PubMed Google Scholar * Mirsadraee, S. et al. Development and
characterization of an acellular human pericardial matrix for tissue engineering. _Tissue Eng._ 12, 763–773 (2006). Article CAS PubMed Google Scholar * Bodnar, E., Olsen, E.G., Florio,
R. & Dobrin, J. Damage of porcine aortic valve tissue caused by the surfactant sodiumdodecylsulphate. _Thorac. Cardiovasc. Surg._ 34, 82–85 (1986). Article CAS PubMed Google Scholar
* Grabow, N. et al. Mechanical and structural properties of a novel hybrid heart valve scaffold for tissue engineering. _Artif. Organs_ 28, 971–979 (2004). Article CAS PubMed Google
Scholar * Russ, J. _The Image Processing Handbook_ Ch 4. (CRC Press, London, 2002). Google Scholar * Press, W.H., Teukolsky, S.A., Vetterling, W.T. & Flannery, B.P. in _Numerical
Recipies in C: The Art of Scientific Computing_ Ch. 12 (Cambridge University Press, Cambridge, UK, 1992). Google Scholar * Ono, K. & Lindsey, E.S. Improved technique of heart
transplantation in rats. _J. Thorac. Cardiovasc. Surg._ 57, 225–229 (1969). CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank S. Keirstead and D. Lowe for access to
electromechanical stimulation equipment and guidance; J. Sedgewick and J. Oja of the Biomedical Image Processing Laboratory at the University of Minnesota, Minneapolis, for access to
photographic equipment and technical support; and the staff of the University of Minnesota CharFac facility, especially A. Ressler, for TEM assistance. This study was supported by a Faculty
Research Development Grant to H.C.O. and D.A.T. from the Academic Health Center, University of Minnesota, Minneapolis, and by funding from the Center for Cardiovascular Repair, University of
Minnesota, and the Medtronic Foundation to D.A.T. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Surgery, Massachusetts General Hospital, Harvard Medical School, 55 Fruit
Street, Boston, 02114, Massachusetts, USA Harald C Ott * Center for Cardiovascular Repair, University of Minnesota, 312 Church Street Southeast, 7-105A NHH, Minneapolis, 55455, Minnesota,
USA Thomas S Matthiesen, Saik-Kia Goh, Stefan M Kren & Doris A Taylor * Department of Biomedical Engineering, University of Minnesota, 312 Church Street Southeast, 7 NHH, Minneapolis,
55455, Minnesota, USA Lauren D Black & Theoden I Netoff * Department of Integrative Biology and Physiology, University of Minnesota, 6-125 Jackson Hall, 312 Church Street Southeast,
Minneapolis, 55455, Minnesota, USA Doris A Taylor Authors * Harald C Ott View author publications You can also search for this author inPubMed Google Scholar * Thomas S Matthiesen View
author publications You can also search for this author inPubMed Google Scholar * Saik-Kia Goh View author publications You can also search for this author inPubMed Google Scholar * Lauren D
Black View author publications You can also search for this author inPubMed Google Scholar * Stefan M Kren View author publications You can also search for this author inPubMed Google
Scholar * Theoden I Netoff View author publications You can also search for this author inPubMed Google Scholar * Doris A Taylor View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS H.C.O. and D.A.T. conceived, designed and oversaw all of the studies, collection of results, interpretation of data and writing of the manuscript.
H.C.O. was responsible for the primary undertaking, completion and supervision of all studies during his tenure at the University of Minnesota. T.S.M. designed and implemented the bioreactor
studies along with H.C.O., participated in the mechanical testing studies and was instrumental in data and figure preparation for the final manuscript. S.-K.G. performed most of the
immunohistochemistry and staining, except for the re-endothelialized tissues. L.D.B. performed the mechanical testing. S.M.K. decellularized the hearts, performed all surgeries and
re-endothelialization experiments, and participated in the bioreactor studies. T.I.N. performed the motion analysis of the movies. CORRESPONDING AUTHOR Correspondence to Doris A Taylor.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY TEXT AND FIGURES Supplementary Figs. 1–3 (PDF 1965 kb) SUPPLEMENTARY MOVIE 1 Heterotopic transplant of decellularized rat heart into RNU rat abdomen.
(MOV 1168 kb) SUPPLEMENTARY MOVIE 2 Recellularization of decellularized heart tissue sections with neonatal cardiomyocytes. (MOV 544 kb) SUPPLEMENTARY MOVIE 3 Recellularized heart construct
with an estimate of wall movement on day 4. (MOV 816 kb) SUPPLEMENTARY MOVIE 4 Recellularized heart construct with an estimate of wall movement on day 4. (MOV 1113 kb) RIGHTS AND PERMISSIONS
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ott, H., Matthiesen, T., Goh, SK. _et al._ Perfusion-decellularized matrix: using nature's platform to engineer a
bioartificial heart. _Nat Med_ 14, 213–221 (2008). https://doi.org/10.1038/nm1684 Download citation * Received: 29 May 2007 * Accepted: 18 October 2007 * Published: 13 January 2008 * Issue
Date: February 2008 * DOI: https://doi.org/10.1038/nm1684 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative