- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Bidirectional cellular communication is integral to both cancer progression and embryological development. In addition, aggressive tumor cells are phenotypically plastic, sharing
many properties with embryonic cells. Owing to the similarities between these two types of cells, the developing zebrafish can be used as a biosensor for tumor-derived signals. Using this
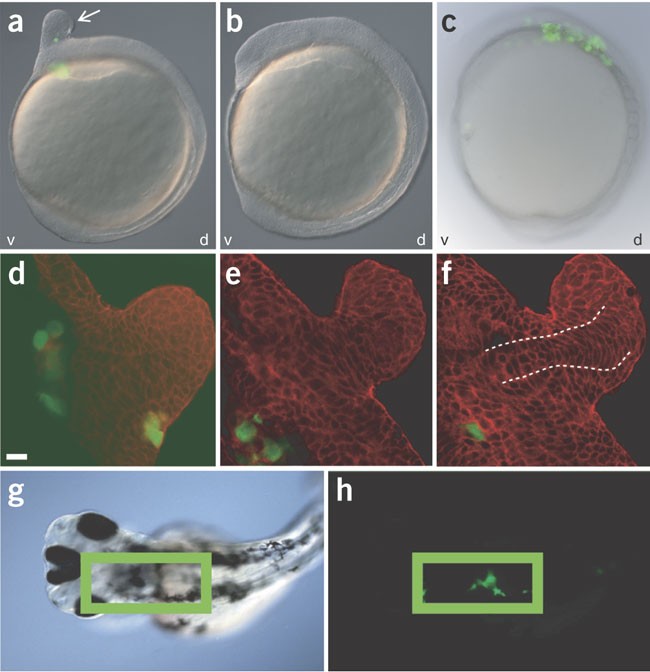
system, we show that aggressive melanoma cells secrete Nodal (a potent embryonic morphogen) and consequently can induce ectopic formation of the embryonic axis. We further show that Nodal is
present in human metastatic tumors, but not in normal skin, and thus may be involved in melanoma pathogenesis. Inhibition of Nodal signaling reduces melanoma cell invasiveness, colony
formation and tumorigenicity. Nodal inhibition also promotes the reversion of melanoma cells toward a melanocytic phenotype. These data suggest that Nodal signaling has a key role in
melanoma cell plasticity and tumorigenicity, thereby providing a previously unknown molecular target for regulating tumor progression. Access through your institution Buy or subscribe This
is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $209.00
per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated
during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS FUNCTIONAL IN
VIVO CHARACTERIZATION OF _SOX10_ ENHANCERS IN NEURAL CREST AND MELANOMA DEVELOPMENT Article Open access 07 June 2021 ANATOMIC POSITION DETERMINES ONCOGENIC SPECIFICITY IN MELANOMA Article 30
March 2022 LOSS OF NECTIN1 TRIGGERS MELANOMA DISSEMINATION UPON LOCAL IGF1 DEPLETION Article Open access 13 October 2022 REFERENCES * Bittner, M. et al. Molecular classification of
cutaneous malignant melanoma by gene expression profiling. _Nature_ 406, 536–540 (2000). CAS PubMed Google Scholar * Hendrix, M.J., Seftor, E.A., Hess, A.R. & Seftor, R.E.
Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. _Nat. Rev. Cancer_ 3, 411–421 (2003). CAS PubMed Google Scholar * Pierce, G.B., Pantazis, C.G., Caldwell, J.E.
& Wells, R.S. Specificity of the control of tumor formation by the blastocyst. _Cancer Res._ 42, 1082–1087 (1982). CAS PubMed Google Scholar * Gerschenson, M., Graves, K., Carson,
S.D., Wells, R.S. & Pierce, G.B. Regulation of melanoma by the embryonic skin. _Proc. Natl. Acad. Sci. USA_ 83, 7307–7310 (1986). CAS PubMed Google Scholar * Lee, L.M., Seftor, E.A.,
Bonde, G., Cornell, R.A. & Hendrix, M.J. The fate of human malignant melanoma cells transplanted into zebrafish embryos: assessment of migration and cell division in the absence of tumor
formation. _Dev. Dyn._ 233, 1560–1570 (2005). CAS PubMed Google Scholar * Mintz, B. & Illmensee, K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells.
_Proc. Natl. Acad. Sci. USA_ 72, 3585–3589 (1975). CAS PubMed Google Scholar * Topczewska, J.M. et al. The winged helix transcription factor Foxc1a is essential for somitogenesis in
zebrafish. _Genes Dev._ 15, 2483–2493 (2001). CAS PubMed PubMed Central Google Scholar * De Robertis, E.M., Larrain, J., Oelgeschlager, M. & Wessely, O. The establishment of
Spemann's organizer and patterning of the vertebrate embryo. _Nat. Rev. Genet._ 1, 171–181 (2000). CAS PubMed PubMed Central Google Scholar * Niehrs, C. Regionally specific
induction by the Spemann-Mangold organizer. _Nat. Rev. Genet._ 5, 425–434 (2004). CAS PubMed Google Scholar * Hatta, K. & Takahashi, Y. Secondary axis induction by heterospecific
organizers in zebrafish. _Dev. Dyn._ 205, 183–195 (1996). CAS PubMed Google Scholar * Gritsman, K., Talbot, W.S. & Schier, A.F. Nodal signaling patterns the organizer. _Development_
127, 921–932 (2000). CAS PubMed Google Scholar * Whitman, M. Nodal signaling in early vertebrate embryos: themes and variations. _Dev. Cell_ 1, 605–617 (2001). CAS PubMed Google Scholar
* Schier, A.F. Nodal signaling in vertebrate development. _Annu. Rev. Cell Dev. Biol._ 19, 589–621 (2003). CAS PubMed Google Scholar * Iannaccone, P.M., Zhou, X., Khokha, M., Boucher,
D. & Kuehn, M.R. Insertional mutation of a gene involved in growth regulation of the early mouse embryo. _Dev. Dyn._ 194, 198–208 (1992). CAS PubMed Google Scholar * Smith, J.C.
Mesoderm-inducing factors and mesodermal patterning. _Curr. Opin. Cell Biol._ 7, 856–861 (1995). CAS PubMed Google Scholar * Zhou, X., Sasaki, H., Lowe, L., Hogan, B.L. & Kuehn, M.R.
Nodal is a novel TGF-β-like gene expressed in the mouse node during gastrulation. _Nature_ 361, 543–547 (1993). CAS PubMed Google Scholar * Rebagliati, M.R., Toyama, R., Haffter, P. &
Dawid, I.B. Cyclops encodes a nodal-related factor involved in midline signaling. _Proc. Natl. Acad. Sci. USA_ 95, 9932–9937 (1998). CAS PubMed Google Scholar * Toyama, R.,
O'Connell, M.L., Wright, C.V., Kuehn, M.R. & Dawid, I.B. Nodal induces ectopic goosecoid and lim1 expression and axis duplication in zebrafish. _Development_ 121, 383–391 (1995).
CAS PubMed Google Scholar * Halpern, M.E. et al. Genetic interactions in zebrafish midline development. _Dev. Biol._ 187, 154–170 (1997). CAS PubMed Google Scholar * Chen, Y. &
Schier, A.F. The zebrafish Nodal signal Squint functions as a morphogen. _Nature_ 411, 607–610 (2001). CAS PubMed Google Scholar * Cheng, S.K., Olale, F., Brivanlou, A.H. & Schier,
A.F. Lefty blocks a subset of TGFβ signals by antagonizing EGF-CFC coreceptors. _PLoS Biol._ 2, 0215–0226 (2004). CAS Google Scholar * Chen, C. & Shen, M.M. Two modes by which Lefty
proteins inhibit nodal signaling. _Curr. Biol._ 14, 618–624 (2004). CAS PubMed Google Scholar * Branford, W.W. & Yost, H.J. Nodal signaling: CrypticLefty mechanism of antagonism
decoded. _Curr. Biol._ 14, R341–R343 (2004). CAS PubMed Google Scholar * Besser, D. Expression of nodal, lefty-a, and lefty-b in undifferentiated human embryonic stem cells requires
activation of Smad2/3. _J. Biol. Chem._ 279, 45076–45084 (2004). CAS PubMed Google Scholar * Hendrix, M.J. et al. Coexpression of vimentin and keratins by human melanoma tumor cells:
correlation with invasive and metastatic potential. _J. Natl. Cancer Inst._ 84, 165–174 (1992). CAS PubMed Google Scholar * Hendrix, M.J., Seftor, E.A., Hess, A.R. & Seftor, R.E.
Molecular plasticity of human melanoma cells. _Oncogene_ 22, 3070–3075 (2003). CAS PubMed Google Scholar * James, D., Levine, A.J., Besser, D. & Hemmati-Brivanlou, A.
TGFβ/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. _Development_ 132, 1273–1282 (2005). CAS PubMed Google Scholar * Vallier, L.,
Reynolds, D. & Pedersen, R.A. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. _Dev. Biol._ 275, 403–421 (2004). CAS PubMed
Google Scholar * Vallier, L., Alexander, M. & Pedersen, R.A. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. _J. Cell Sci._ 118,
4495–4509 (2005). CAS PubMed Google Scholar * Hendrix, M.J., Seftor, E.A., Kirschmann, D.A., Quaranta, V. & Seftor, R.E. Remodeling of the microenvironment by aggressive melanoma
tumor cells. _Ann. NY Acad. Sci._ 995, 151–161 (2003). CAS PubMed Google Scholar * Seftor, E.A. et al. Epigenetic transformation of normal melanocytes by a metastatic melanoma
microenvironment. _Cancer Res._ 65, 10164–10169 (2005). CAS PubMed Google Scholar * Chu, Y.W., Seftor, E.A., Romer, L.H. & Hendrix, M.J. Experimental coexpression of vimentin and
keratin intermediate filaments in human melanoma cells augments motility. _Am. J. Pathol._ 148, 63–69 (1996). CAS PubMed PubMed Central Google Scholar * Hendrix, M.J. et al. Expression
and functional significance of VE-cadherin in aggressive human melanoma cells: role in vasculogenic mimicry. _Proc. Natl. Acad. Sci. USA_ 98, 8018–8023 (2001). CAS PubMed Google Scholar *
Takeuchi, H., Kuo, C., Morton, D.L., Wang, H.J. & Hoon, D.S. Expression of differentiation melanoma-associated antigen genes is associated with favorable disease outcome in
advanced-stage melanomas. _Cancer Res._ 63, 441–448 (2003). CAS PubMed Google Scholar * Martinez-Esparza, M., Solano, F. & Garcia-Borron, J.C. Independent regulation of tyrosinase by
the hypopigmenting cytokines TGF β1 and TNF α and the melanogenic hormone α-MSH in B16 mouse melanocytes. _Cell. Mol. Biol._ 45, 991–1000 (1999). CAS PubMed Google Scholar * Kim, D.S.,
Park, S.H. & Park, K.C. Transforming growth factor-β1 decreases melanin synthesis via delayed extracellular signal-regulated kinase activation. _Int. J. Biochem. Cell Biol._ 36,
1482–1491 (2004). CAS PubMed Google Scholar * Nawshad, A., Lagamba, D., Polad, A. & Hay, E.D. Transforming growth factor-β signaling during epithelial-mesenchymal transformation:
implications for embryogenesis and tumor metastasis. _Cells Tissues Organs_ 179, 11–23 (2005). CAS PubMed Google Scholar * Javelaud, D. et al. Stable overexpression of Smad7 in human
melanoma cells inhibits their tumorigenicity _in vitro_ and _in vivo_. _Oncogene_ 24, 7624–7629 (2005). CAS PubMed Google Scholar * Juhasz, I. et al. Growth and invasion of human
melanomas in human skin grafted to immunodeficient mice. _Am. J. Pathol._ 143, 528–537 (1993). CAS PubMed PubMed Central Google Scholar * Adkins, H.B. et al. Antibody blockade of the
Cripto CFC domain suppresses tumor cell growth _in vivo_. _J. Clin. Invest._ 112, 575–587 (2003). CAS PubMed PubMed Central Google Scholar * Reya, T., Morrison, S.J., Clarke, M.F. &
Weissman, I.L. Stem cells, cancer, and cancer stem cells. _Nature_ 414, 105–111 (2001). CAS Google Scholar * Fang, D. et al. A tumorigenic subpopulation with stem cell properties in
melanomas. _Cancer Res._ 65, 9328–9337 (2005). CAS PubMed Google Scholar * Hendrix, M.J. et al. Transendothelial function of human metastatic melanoma cells: role of the microenvironment
in cell-fate determination. _Cancer Res._ 62, 665–668 (2002). CAS PubMed Google Scholar * Welch, D.R. et al. Characterization of a highly invasive and spontaneously metastatic human
malignant melanoma cell line. _Int. J. Cancer_ 47, 227–237 (1991). CAS PubMed Google Scholar * Seftor, E.A. et al. Expression of multiple molecular phenotypes by aggressive melanoma tumor
cells: role in vasculogenic mimicry. _Crit. Rev. Oncol. Hematol._ 44, 17–27 (2002). PubMed Google Scholar * Solnica-Krezel, L., Schier, A.F. & Driever, W. Efficient recovery of
ENU-induced mutations from the zebrafish germline. _Genetics_ 136, 1401–1420 (1994). CAS PubMed PubMed Central Google Scholar * Thisse, C., Thisse, B., Schilling, T.F. &
Postlethwait, J.H. Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. _Development_ 119, 1203–1215 (1993). CAS PubMed Google
Scholar * Hess, A.R. et al. Molecular regulation of tumor cell vasculogenic mimicry by tyrosine phosphorylation: role of epithelial cell kinase (Eck/EphA2). _Cancer Res._ 61, 3250–3255
(2001). CAS PubMed Google Scholar * Hendrix, M.J., Seftor, E.A., Seftor, R.E. & Fidler, I.J. A simple quantitative assay for studying the invasive potential of high and low human
metastatic variants. _Cancer Lett._ 38, 137–147 (1987). CAS PubMed Google Scholar * Maniotis, A.J. et al. Vascular channel formation by human melanoma cells _in vivo_ and _in vitro_:
vasculogenic mimicry. _Am. J. Pathol._ 155, 739–752 (1999). CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We acknowledge discussions with D. Constam and
the input of L. Lee, E. Seftor and R. Seftor. This work was supported in part by US National Institutes of Health (NIH) grants (CA59702 and CA80318), an Illinois Regenerative Medicine
Institute grant and a Charlotte Geyer Foundation grant to M.J.C.H.; an Excellence in Academic Medicine grant (FY05EAM) to J.M.T.; Mazza Foundation grants to J.M.T. and M.J.C.H.; NIH grants
(CA59327 and CA27502) to B.J.N.; and a Canadian Institutes of Health Research postdoctoral fellowship to L.M.P. AUTHOR INFORMATION Author notes * Jolanta M Topczewska and Lynne-Marie
Postovit: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Program in Developmental Biology, Children's Memorial Research Center, Feinberg School of Medicine
Northwestern University, 2300 Children's Plaza, Box 222, Chicago, 60614, Illinois, USA Jolanta M Topczewska, Anthony Sam & Jacek Topczewski * Program in Cancer Biology and
Epigenomics, Children's Memorial Research Center, Feinberg School of Medicine Northwestern University, 2300 Children's Plaza, Box 222, Chicago, 60614, Illinois, USA Lynne-Marie
Postovit, Naira V Margaryan, Angela R Hess, William W Wheaton & Mary J C Hendrix * Department of Pathology, Loyola University Medical Center, Cardinal Bernardin Cancer Center, 2160 S.
First Avenue, Building 112, Room 301, Maywood, 60153, Illinois, USA Brian J Nickoloff Authors * Jolanta M Topczewska View author publications You can also search for this author inPubMed
Google Scholar * Lynne-Marie Postovit View author publications You can also search for this author inPubMed Google Scholar * Naira V Margaryan View author publications You can also search
for this author inPubMed Google Scholar * Anthony Sam View author publications You can also search for this author inPubMed Google Scholar * Angela R Hess View author publications You can
also search for this author inPubMed Google Scholar * William W Wheaton View author publications You can also search for this author inPubMed Google Scholar * Brian J Nickoloff View author
publications You can also search for this author inPubMed Google Scholar * Jacek Topczewski View author publications You can also search for this author inPubMed Google Scholar * Mary J C
Hendrix View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.M.T. carried out the transplantations and RNA injections into zebrafish embryos,
whole-mount immunohistochemistry, and confocal and conventional imaging. L.-M.P. did the cell culture, adenoviral transfections, morpholino treatments, cell sorting, clonogenic assays,
vasculogenic mimicry assays, mouse studies and western blotting. N.V.M. carried out the immunohistochemical tissue staining in conjunction with B.J.N. and L.-M.P., and did the mouse studies
with L.M.P. A.S. carried out fish care and _in situ_ hybridization, and A.R.H. completed the invasion assays. L.-M.P. and J.M.T. wrote the paper, and all authors discussed the results and
commented on the manuscript. The project was conceived and orchestrated by M.J.C.H. _Note: __Supplementary information_ _ is available on the Nature Medicine website_. CORRESPONDING AUTHOR
Correspondence to Mary J C Hendrix. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIG. 1 Schematic
representation of melanoma cell transplantation experiments. (PDF 529 kb) SUPPLEMENTARY FIG. 2 The overexpression of the lefty1 inhibits secondary axis formation induced by transplanted
aggressive C8161 melanoma cells. (PDF 2537 kb) SUPPLEMENTARY FIG. 3 Patterns of Nodal expression in primary and metastatic cutaneous melanoma and breast carcinoma cell lines. (PDF 433 kb)
SUPPLEMENTARY FIG. 4 Nodal-mediated signaling and gene regulation in melanoma cells. (PDF 103 kb) SUPPLEMENTARY FIG. 5 TUNEL assay of melanoma cells treated with MONodal or SB431542. (PDF
685 kb) SUPPLEMENTARY NOTE (PDF 430 KB) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Topczewska, J., Postovit, LM., Margaryan, N. _et al._ Embryonic
and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. _Nat Med_ 12, 925–932 (2006). https://doi.org/10.1038/nm1448 Download citation * Received: 02 February
2005 * Accepted: 21 June 2006 * Published: 30 July 2006 * Issue Date: 01 August 2006 * DOI: https://doi.org/10.1038/nm1448 SHARE THIS ARTICLE Anyone you share the following link with will
be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative