- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
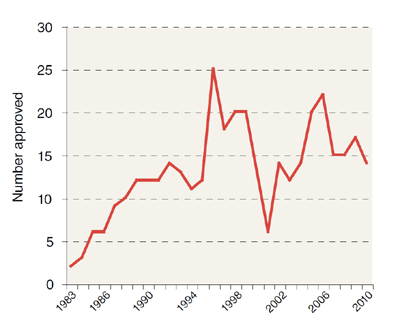
Access through your institution Buy or subscribe In 1983, US lawmakers passed the Orphan Drug Act to encourage pharmaceutical companies to pursue treatments for largely ignored diseases
affecting small populations. And for the next 15 years or so, the number of rare diseases given orphan drug status hovered between about 40 and 80 per year. But over the last decade, that
number began steadily increasing, and last year the US Food and Drug Administration (FDA) granted a record 192 designations. Developing orphan drugs “makes a lot of business sense if you
don't have a blockbuster,” says Syamala Ariyanchira, an independent Malaysia-based pharmaceutical consultant who estimates that the orphan drug market will exceed $80 billion this year.
This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access
$209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are
calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support Authors * Monica Heger View author
publications You can also search for this author inPubMed Google Scholar RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Heger, M. Despite surge in
orphan drug designations, approvals still lag. _Nat Med_ 17, 236 (2011). https://doi.org/10.1038/nm0311-236b Download citation * Published: 07 March 2011 * Issue Date: March 2011 * DOI:
https://doi.org/10.1038/nm0311-236b SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative








