- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Oligodendrocytes myelinate axons for rapid impulse conduction and contribute to normal axonal functions in the central nervous system. In multiple sclerosis, demyelination is caused
by autoimmune attacks, but the role of oligodendroglial cells in disease progression and axon degeneration is unclear. Here we show that oligodendrocytes harbor peroxisomes whose function
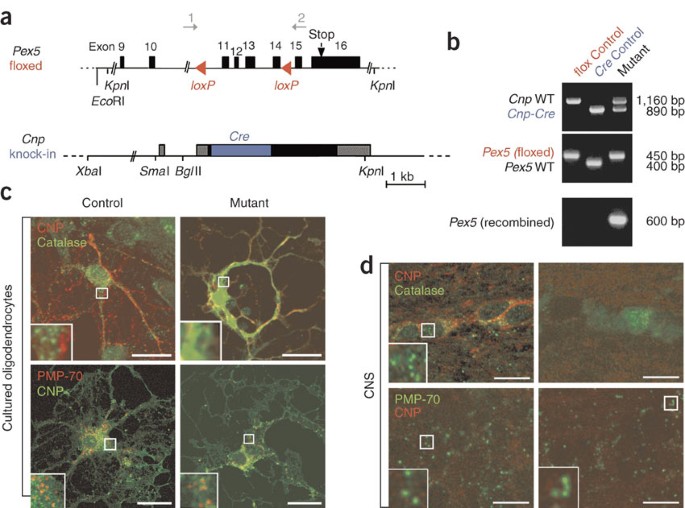
is essential for maintaining white matter tracts throughout adult life. By selectively inactivating the import factor PEX5 in myelinating glia, we generated mutant mice that developed
normally, but within several months showed ataxia, tremor and premature death. Absence of functional peroxisomes from oligodendrocytes caused widespread axonal degeneration and progressive
subcortical demyelination, but did not interfere with glial survival. Moreover, it caused a strong proinflammatory milieu and, unexpectedly, the infiltration of B and activated CD8+ T cells
into brain lesions. We conclude that peroxisomes provide oligodendrocytes with an essential neuroprotective function against axon degeneration and neuroinflammation, which is relevant for
human demyelinating diseases. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your
institution Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access
to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read
our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS REMYELINATION PROTECTS NEURONS FROM DLK-MEDIATED NEURODEGENERATION Article Open access 23 October 2024
MICROGLIA-MEDIATED DEMYELINATION PROTECTS AGAINST CD8+ T CELL-DRIVEN AXON DEGENERATION IN MICE CARRYING PLP DEFECTS Article Open access 30 October 2023 DOR ACTIVATION IN MATURE
OLIGODENDROCYTES REGULATES Α-KETOGLUTARATE METABOLISM LEADING TO ENHANCED REMYELINATION IN AGED MICE Article 12 September 2024 REFERENCES * Lazzarini, R.A. _Myelin Biology and Disorders_
(Elsevier Academic, San Diego, California, USA, 2004). Google Scholar * Bjartmar, C. & Trapp, B.D. Axonal and neuronal degeneration in multiple sclerosis: mechanisms and functional
consequences. _Curr. Opin. Neurol._ 14, 271–278 (2001). Article CAS Google Scholar * Trapp, B.D. et al. Axonal transection in the lesions of multiple sclerosis. _N. Engl. J. Med._ 338,
278–285 (1998). Article CAS Google Scholar * Griffiths, I. et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. _Science_ 280, 1610–1613 (1998).
Article CAS Google Scholar * Lappe-Siefke, C. et al. Disruption of _Cnp1_ uncouples oligodendroglial functions in axonal support and myelination. _Nat. Genet._ 33, 366–374 (2003). Article
CAS Google Scholar * Suter, U. & Scherer, S.S. Disease mechanisms in inherited neuropathies. _Nat. Rev. Neurosci._ 4, 714–726 (2003). Article CAS Google Scholar * Edgar, J.M. et
al. Oligodendroglial modulation of fast axonal transport in a mouse model of hereditary spastic paraplegia. _J. Cell Biol._ 166, 121–131 (2004). Article CAS Google Scholar * Ferreirinha,
F. et al. Axonal degeneration in paraplegin-deficient mice is associated with abnormal mitochondria and impairment of axonal transport. _J. Clin. Invest._ 113, 231–242 (2004). Article CAS
Google Scholar * Schrader, M. & Fahimi, H.D. Mammalian peroxisomes and reactive oxygen species. _Histochem. Cell Biol._ 122, 383–393 (2004). Article CAS Google Scholar * Wanders,
R.J. Peroxisomes, lipid metabolism, and peroxisomal disorders. _Mol. Genet. Metab._ 83, 16–27 (2004). Article CAS Google Scholar * Baes, M. et al. A mouse model for Zellweger syndrome.
_Nat. Genet._ 17, 49–57 (1997). Article CAS Google Scholar * Hirrlinger, J., Resch, A., Gutterer, J.M. & Dringen, R. Oligodendroglial cells in culture effectively dispose of exogenous
hydrogen peroxide: comparison with cultured neurones, astroglial and microglial cells. _J. Neurochem._ 82, 635–644 (2002). Article CAS Google Scholar * Gould, S.J. & Collins, C.S.
Opinion: peroxisomal-protein import: is it really that complex? _Nat. Rev. Mol. Cell Biol._ 3, 382–389 (2002). Article CAS Google Scholar * Dodt, G. et al. Mutations in the PTS1 receptor
gene, _PXR1_, define complementation group 2 of the peroxisome biogenesis disorders. _Nat. Genet._ 9, 115–125 (1995). Article CAS Google Scholar * Baes, M., Dewerchin, M., Janssen, A.,
Collen, D. & Carmeliet, P. Generation of _Pex5-loxP_ mice allowing the conditional elimination of peroxisomes. _Genesis_ 32, 177–178 (2002). Article CAS Google Scholar * Chang, C.C.
et al. Metabolic control of peroxisome abundance. _J. Cell Sci._ 112, 1579–1590 (1999). CAS Google Scholar * Saher, G. et al. High cholesterol level is essential for myelin membrane
growth. _Nat. Neurosci._ 8, 468–475 (2005). Article CAS Google Scholar * Zoeller, R.A. & Raetz, C.R. Isolation of animal cell mutants deficient in plasmalogen biosynthesis and
peroxisome assembly. _Proc. Natl. Acad. Sci. USA_ 83, 5170–5174 (1986). Article CAS Google Scholar * Rodemer, C. et al. Inactivation of ether lipid biosynthesis causes male infertility,
defects in eye development and optic nerve hypoplasia in mice. _Hum. Mol. Genet._ 12, 1881–1895 (2003). Article CAS Google Scholar * Moser, H.W., Bergin, A. & Cornblath, D.
Peroxisomal disorders. _Biochem. Cell Biol._ 69, 463–474 (1991). Article CAS Google Scholar * Wanders, R.J. & Waterham, H.R. Peroxisomal disorders I: biochemistry and genetics of
peroxisome biogenesis disorders. _Clin. Genet._ 67, 107–133 (2005). Article CAS Google Scholar * Forss-Petter, S. et al. Targeted inactivation of the X-linked adrenoleukodystrophy gene in
mice. _J. Neurosci. Res._ 50, 829–843 (1997). Article CAS Google Scholar * Lu, J.F. et al. A mouse model for X-linked adrenoleukodystrophy. _Proc. Natl. Acad. Sci. USA_ 94, 9366–9371
(1997). Article CAS Google Scholar * Kobayashi, T., Shinnoh, N., Kondo, A. & Yamada, T. Adrenoleukodystrophy protein-deficient mice represent abnormality of very long chain fatty acid
metabolism. _Biochem. Biophys. Res. Commun._ 232, 631–636 (1997). Article CAS Google Scholar * Simpson, J.E., Newcombe, J., Cuzner, M.L. & Woodroofe, M.N. Expression of monocyte
chemoattractant protein-1 and other beta-chemokines by resident glia and inflammatory cells in multiple sclerosis lesions. _J. Neuroimmunol._ 84, 238–249 (1998). Article CAS Google Scholar
* McManus, C. et al. MCP-1, MCP-2 and MCP-3 expression in multiple sclerosis lesions: an immunohistochemical and in situ hybridization study. _J. Neuroimmunol._ 86, 20–29 (1998). Article
CAS Google Scholar * Loers, G., Aboul-Enein, F., Bartsch, U., Lassmann, H. & Schachner, M. Comparison of myelin, axon, lipid, and immunopathology in the central nervous system of
differentially myelin-compromised mutant mice: a morphological and biochemical study. _Mol. Cell. Neurosci._ 27, 175–189 (2004). Article CAS Google Scholar * Genoud, S. et al. Notch1
control of oligodendrocyte differentiation in the spinal cord. _J. Cell Biol._ 158, 709–718 (2002). Article CAS Google Scholar * Schrader, M. & Fahimi, H.D. Peroxisomes and oxidative
stress. _Biochim. Biophys. Acta_ 1763, 1755–1766 (2006). Article CAS Google Scholar * Loes, D.J. et al. Analysis of MRI patterns aids prediction of progression in X-linked
adrenoleukodystrophy. _Neurology_ 61, 369–374 (2003). Article CAS Google Scholar * Wilken, B. et al. Quantitative proton magnetic resonance spectroscopy of children with
adrenoleukodystrophy before and after hematopoietic stem cell transplantation. _Neuropediatrics_ 34, 237–246 (2003). Article CAS Google Scholar * Dubois-Dalcq, M., Feigenbaum, V. &
Aubourg, P. The neurobiology of X-linked adrenoleukodystrophy, a demyelinating peroxisomal disorder. _Trends Neurosci._ 22, 4–12 (1999). Article CAS Google Scholar * Mosser, J. et al.
Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. _Nature_ 361, 726–730 (1993). Article CAS Google Scholar * Kemp, S. & Wanders, R.J.
X-linked adrenoleukodystrophy: Very long-chain fatty acid metabolism, ABC half-transporters and the complicated route to treatment. _Mol. Genet. Metab._ 90, 268–276 (2007). Article CAS
Google Scholar * Pujol, A. et al. Late onset neurological phenotype of the X-ALD gene inactivation in mice: a mouse model for adrenomyeloneuropathy. _Hum. Mol. Genet._ 11, 499–505 (2002).
Article CAS Google Scholar * Hudspeth, M.P. & Raymond, G.V. Immunopathogenesis of adrenoleukodystrophy: current understanding. _J. Neuroimmunol._ 182, 5–12 (2006). Article Google
Scholar * Barth, P.G. et al. Late onset white matter disease in peroxisome biogenesis disorder. _Neurology_ 57, 1949–1955 (2001). Article CAS Google Scholar * Baumgartner, M.R. et al.
Clinical approach to inherited peroxisomal disorders: a series of 27 patients. _Ann. Neurol._ 44, 720–730 (1998). Article CAS Google Scholar * Goverman, J. et al. The role of CD8+ T cells
in multiple sclerosis and its animal models. _Curr. Drug Targets Inflamm. Allergy_ 4, 239–245 (2005). Article CAS Google Scholar * Barnett, M.H. & Prineas, J.W. Relapsing and
remitting multiple sclerosis: pathology of the newly forming lesion. _Ann. Neurol._ 55, 458–468 (2004). Article Google Scholar * Raivich, G. et al. Immune surveillance in the injured
nervous system: T-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuronal degeneration. _J. Neurosci._ 18, 5804–5816 (1998). Article CAS Google
Scholar * Ip, C.W. et al. Immune cells contribute to myelin degeneration and axonopathic changes in mice overexpressing proteolipid protein in oligodendrocytes. _J. Neurosci._ 26, 8206–8216
(2006). Article CAS Google Scholar * Natt, O. et al. High-resolution 3D MRI of mouse brain reveals small cerebral structures in vivo. _J. Neurosci. Methods_ 120, 203–209 (2002). Article
CAS Google Scholar * Gallyas, F. Silver staining of myelin by means of physical development. _Neurol. Res._ 1, 203–209 (1979). Article CAS Google Scholar * Bielschowsky, M. Die
Silberimprägnation der Neurofibrillen. _J. Psychol. Neurol._ 3, 169–183 (1904). Google Scholar * Jung, M., Sommer, I., Schachner, M. & Nave, K.A. Monoclonal antibody O10 defines a
conformationally sensitive cell-surface epitope of proteolipid protein (PLP): evidence that PLP misfolding underlies dysmyelination in mutant mice. _J. Neurosci._ 16, 7920–7929 (1996).
Article CAS Google Scholar * Norton, W.T. & Poduslo, S.E. Myelination in rat brain: method of myelin isolation. _J. Neurochem._ 21, 749–757 (1973). Article CAS Google Scholar *
Bligh, E.G. & Dyer, W.J. A rapid method of total lipid extraction and purification. _Can. J. Biochem. Physiol._ 37, 911–917 (1959). Article CAS Google Scholar * Koivusalo, M., Haimi,
P., Heikinheimo, L., Kostiainen, R. & Somerharju, P. Quantitative determination of phospholipid compositions by ESI-MS: effects of acyl chain length, unsaturation, and lipid
concentration on instrument response. _J. Lipid Res._ 42, 663–672 (2001). Google Scholar * Gutcher, I., Urich, E., Wolter, K., Prinz, M. & Becher, B. Interleukin 18–independent
engagement of interleukin 18 receptor is required for autoimmune inflammation. _Nat. Immunol._ 7, 946–953 (2006). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We
dedicate this paper to Hugo Moser for his pioneering work on peroxisomes in childhood neurological diseases. We thank J. Barth, U. Bode, A. Fahrenholz, A. Nave, S. Relitz and S. Hühold for
excellent technical assistance, and gratefully acknowledge J. Gärtner and D.H. Hunneman for clinical diagnostic service (VLCFA). This work was funded by grants from the European Union (PEX
and X-ALD), the US National Multiple Sclerosis Society, the Hertie Foundation, and the generous support of the private Liley and Del Marmol Foundations. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Department of Neurogenetics, Max Planck Institute of Experimental Medicine, Hermann-Rein-Strasse 3, Göttingen, D-37075, Germany Celia M Kassmann, Corinna Lappe-Siefke, Hauke B
Werner & Klaus-Armin Nave * Laboratory for Cell Metabolism, Faculty of Pharmacy, Katholieke Universiteit Leuven, Leuven, 3000, Belgium Myriam Baes * Biochemie-Zentrum, Universität
Heidelberg, Heidelberg, D-69120, Germany Britta Brügger * Department of Neuropathology, University of Göttingen, Göttingen, D-37075, Germany Alexander Mildner & Marco Prinz *
Biomedizinische NMR Forschungs GmbH am Max-Planck-Institut für biophysikalische Chemie, Göttingen, 37077, Germany Oliver Natt, Thomas Michaelis & Jens Frahm Authors * Celia M Kassmann
View author publications You can also search for this author inPubMed Google Scholar * Corinna Lappe-Siefke View author publications You can also search for this author inPubMed Google
Scholar * Myriam Baes View author publications You can also search for this author inPubMed Google Scholar * Britta Brügger View author publications You can also search for this author
inPubMed Google Scholar * Alexander Mildner View author publications You can also search for this author inPubMed Google Scholar * Hauke B Werner View author publications You can also search
for this author inPubMed Google Scholar * Oliver Natt View author publications You can also search for this author inPubMed Google Scholar * Thomas Michaelis View author publications You
can also search for this author inPubMed Google Scholar * Marco Prinz View author publications You can also search for this author inPubMed Google Scholar * Jens Frahm View author
publications You can also search for this author inPubMed Google Scholar * Klaus-Armin Nave View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
C.M.K. performed the analyses and drafted the manuscript. C.L.-S. provided _Cnp-Cre_ and M.B. provided _Pex5_ floxed mice. B.B. determined myelin lipids by mass spectroscopy. A.M. and M.P.
performed FACS analysis and quantified cytokines. H.B.W. participated in mouse genetics. O.N., T.M. and J.F. performed MRI. K.-A.N. designed the study and finalized the manuscript.
CORRESPONDING AUTHOR Correspondence to Klaus-Armin Nave. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY
TEXT AND FIGURES Supplementary Figures 1–4 (PDF 731 kb) SUPPLEMENTARY VIDEO 1 Ataxia of _Pex5__flox/flox*__Cnp1-Cre_ mice. Conditional _Pex5_ mutant mouse (_Pex5__flox/flox*__Cnp1-Cre_)
with hind limb ataxia as found in clinical stage III-IV. Motor defects are obvious when the mouse is placed on a cage top (slow motion video). (MOV 1096 kb) RIGHTS AND PERMISSIONS Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kassmann, C., Lappe-Siefke, C., Baes, M. _et al._ Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. _Nat
Genet_ 39, 969–976 (2007). https://doi.org/10.1038/ng2070 Download citation * Received: 11 January 2007 * Accepted: 14 May 2007 * Published: 22 July 2007 * Issue Date: August 2007 * DOI:
https://doi.org/10.1038/ng2070 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative