- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Deposition of α-synuclein aggregates occurs widely in the central and peripheral nervous systems in Parkinson’s disease (PD). Although recent evidence has suggested that
cell-to-cell transmission of α-synuclein aggregates is associated with the progression of PD, the mechanism by which α-synuclein aggregates spread remains undefined. Here, we show that
α-synuclein aggregates are transmitted from cell to cell through a cycle involving uptake of external aggregates, co-aggregation with endogenous α-synuclein and exocytosis of the
co-aggregates. Moreover, we find that glucocerebrosidase depletion, which has previously been strongly associated with PD and increased cognitive impairment, promotes propagation of
α-synuclein aggregates. These studies define how α-synuclein aggregates spread among neuronal cells and may provide an explanation for how glucocerebrosidase mutations increase the risk of
developing PD and other synucleinopathies. You have full access to this article via your institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS MITOCHONDRIAL OXIDANT STRESS
PROMOTES Α-SYNUCLEIN AGGREGATION AND SPREADING IN MICE WITH MUTATED GLUCOCEREBROSIDASE Article Open access 11 December 2024 DOPAL INITIATES ΑSYNUCLEIN-DEPENDENT IMPAIRED PROTEOSTASIS AND
DEGENERATION OF NEURONAL PROJECTIONS IN PARKINSON’S DISEASE Article Open access 25 March 2023 REDUCED PROGRANULIN INCREASES TAU AND Α-SYNUCLEIN INCLUSIONS AND ALTERS MOUSE TAUOPATHY
PHENOTYPES VIA GLUCOCEREBROSIDASE Article Open access 16 February 2024 INTRODUCTION Abnormal deposition of α-synuclein aggregates is a pathological feature of Parkinson’s disease (PD)1.
While a large body of recent studies suggests that transcellular transmission of α-synuclein aggregates is associated with the progression of PD2,3,4,5, the mechanisms underlying such
transmission are not clearly understood. Particularly urgent issues include whether cell-to-cell transmission of aggregates is seeding-dependent, whether the aggregates disseminates to large
cell populations through continuous transmission, and the role of other PD-related genes in this process6. Genetic and pathological evidence has suggested that lysosomal impairment is a
major contributor in the pathogenesis of Lewy body diseases7. The _GBA1_ gene encodes a lysosomal hydrolase, glucocerebrosidase (GCase), which is deficient in Gaucher disease, the most
common lysosomal storage disease. Moreover, mutations in _GBA1_ are strong genetic risk factors in PD8 and in dementia with Lewy bodies9, although the mechanism by which mutations in _GBA1_
increase the risk of PD remains unclear. α-Synuclein aggregates that were transferred from cell to cell were transported through the endolysosomal pathway and were degraded in
lysosomes10,11, the finding that prompted us to hypothesize that _GBA1_ deficiency causes lysosomal dysfunction, thereby increasing the efficiency of aggregate transmission. In the current
study, we investigated the mechanism of transmission of α-synuclein aggregates through continuous cell-to-cell transmission and the roles of GCase, a lysosomal enzyme whose mutations
represent the most common genetic risk for PD and are implicated in disease severity. RESULTS LIVE CELL MODEL FOR AGGREGATE TRANSMISSION To clarify the mechanism of aggregate propagation by
direct observation of cell-to-cell transmission of α-synuclein aggregates, we developed an assay based on bimolecular fluorescence complementation (BiFC), which has been previously
successfully applied to assess protein–protein interactions and protein dimerization or oligomerization in living mammalian cells12. We produced two stable cell lines expressing α-synuclein
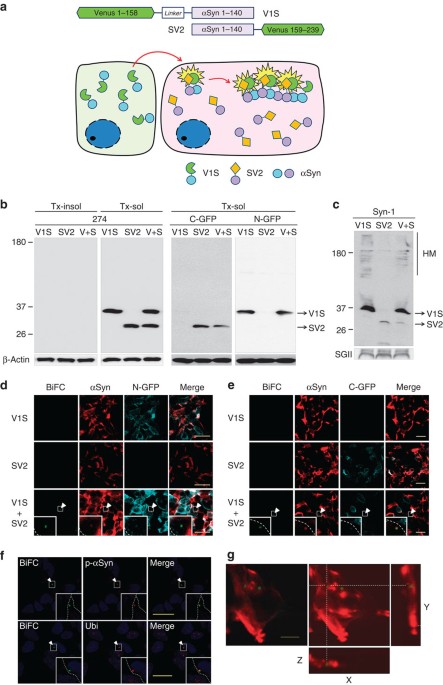
fused to either the amino (N) terminus (V1S) or carboxy (C) terminus (SV2) fragment of Venus, a variant of yellow fluorescence protein (Fig. 1a). The V1S and SV2 constructs were individually
transfected into SH-SY5Y cells, and stable cell lines expressing similar levels of the two α-synuclein fusion proteins were selected (Fig. 1b). As anticipated, neither V1S-expressing cells
nor SV2-expressing cells fluoresced in individual culture (Fig. 1d,e). When the cell lines were cocultured, however, fluorescence resulting from dimerization or oligomerization of the V1S
and SV2 fusion proteins13 during cell-to-cell transfer of α-synuclein was visualized using BiFC (Fig. 1a,d,e). Neither the coculture of cells expressing V1S and the C-terminal fragment of
Venus (V2) nor those expressing SV2 and the N-terminal fragment of Venus (V1) produced BiFC frourescence (Supplementary Fig. 1), validating the specificity of homotypic interaction between
α-synuclein proteins. Since V1S was secreted at a higher level than SV2 (Fig. 1c), transfer of α-synuclein during coculture of the cell lines was assumed to primarily involve V1S.
Immunoflourescence analysis showed that approximately 2–5% of cells contained small fluorescent inclusion bodies positive for α-synuclein and the N and C termini of Venus (Fig. 1d,e),
phospho-α-synuclein (Ser129) and ubiquitin (Fig. 1f). These characteristics are similar to the Lewy bodies and pathogenic inclusions observed in transgenic models14,15,16. Three-dimensional
reconstruction of z-stack images indicated that the fluorescent inclusions were intracellular structures (Fig. 1g). These results collectively demonstrated the cell-to-cell transfer and
co-aggregation of the transferred α-synuclein with resident α-synuclein in the recipient cells, supporting the ‘seeding’ hypothesis of aggregate propagation3. Assessed by western analysis,
the majority of intracellular α-synuclein in V1S and SV2 cells was Triton X-100 soluble and monomeric (Fig. 1b). However, the cell culture media contained the aggregates of α-synuclein (Fig.
1c), suggesting the aggregates were preferentially secreted from cells. This is consistent with our previous findings reported by Kim _et al_.17 and Jang _et al_.18 To further validate the
presence of aggregates in the culture media, we performed size-exclusion chromatography using the total culture medium (V1S CM) and the culture medium passed through a 100 kDa cutoff filter
(V1S CM-FT) (Fig. 2a–c). Histogram of V1S CM showed distribution of α-synuclein in a wide size ranges from monomer (13 ml) to void volume fractions (8 ml), while that of V1S CM-FT showed
only the monomer (Fig. 2b,c). This suggests that the V1S CM contained aggregated forms, and 100 kDa cutoff filtration effectively removed the aggregates, leaving only the monomers. To
confirm the seeded aggregation in the recipient cells, we treated SV2 cells with either the total V1S CM or V1S CM-FT and analysed BiFC-positive aggregates. Administration of the total V1S
CM resulted in BiFC-positive aggregates in SV2 cells, whereas removal of high-molecular weight aggregates from the V1S CM (V1S CM-FT) eliminated the ‘seeding’ ability of the CM (Fig. 2d,e).
These data strongly suggest that cells release aggregated α-synuclein, and the aggregated forms can seed the aggregation in the recipient cells in our dual-cell BiFC model. CONTINUOUS
CELL-TO-CELL TRANSMISSION OF Α-SYNUCLEIN AGGREGATES To explain pathological aggregate spreading within the central nervous system, cell-to-cell transmission should not be a single
discontinuous process. The secondary release of co-aggregated α-synuclein that is produced in the first round of transmission, is absolutely required for spreading of aggregate pathology. To
address this problem, we used the dual-cell BiFC system. During continuous subculture of V1S and SV2-expressing cells, if transmission were a single discontinuous event, the percentage of
BiFC-positive cells would decrease as passage number increased. Conversely, if the transmission were a continuous event, the percentage of BiFC-positive cells would increase with passage
number until reaching a steady state (Supplementary Fig. 5). Coculture of V1S and SV2 cells for several passages (cultured for 48 h for each passage) resulted in a continuous increase in the
percentage of BiFC-positive cells (Fig. 3a,b). Similarly, BiFC fluorescence in the culture media, representing the secondary secretion of co-aggregates of the ‘seed’ and endogenous
α-synuclein, also increased with increasing number of passages (Fig. 3c). This was confirmed by increased quantities of oligomers in the media (Fig. 3d). We also confirmed that the ratio of
BiFC-positive cells between V1S and SV2 cells does not change significantly during the successive subcultures (Supplementary Fig. 6). To demonstrate the transfer of seeds through the fluid
phase, we next performed media washing and antibody blocking experiments. During the coculture of V1S and SV2 cells, culture media were removed and replaced with the fresh media. When BiFC
fluorescence was analysed 1 day after the media replacement, both intracellular and media BiFC signals were decreased (Fig. 3e,f), consistent with fluid phase transfer of the seed. Next, we
added Ab274, an α-synuclein-specific antibody, to the coculture 1 day before the BiFC analysis, to hijack the secreted α-synuclein, thereby blocking the transfer of this protein. This
antibody treatment suppressed BiFC signals in both the cytoplasm and the media (Fig. 3g,h). To assess the temporal changes of the seeding and secondary secretion, we conducted a pulse-chase
experiment, in which conditioned medium (CM) obtained from the V1S culture was added to the SV2 cells. After a steady state was reached, the V1S CM was washed out and the BiFC signal was
analysed in cells and the medium at selected time points. Co-aggregates of V1S and SV2 proteins disappeared rapidly from the cytoplasm after medium washing, whereas the secreted BiFC signal
increased reciprocally (Fig. 3i,j). Oligomer-specific enzyme-linked immunosorbent assay (ELISA) confirmed the increase in the level of α-synuclein oligomers in the medium (Fig. 3k).
Together, these results suggest that α-synuclein aggregates are transferred from cell to cell contiguously through a cycle of sequential events, involving intercellular aggregate transfer,
seeding of the aggregation of endogenous α-synuclein, and secondary secretion of the seeded aggregates (Supplementary Fig. 7). _GBA1_ DEFICIENCY LED TO LYSOSOMAL DYSFUNCTION To assess the
role of _GBA1_, a strong genetic risk factor for PD, in α-synuclein aggregate transmission, we used a zinc-finger nuclease-based method to establish an SV2 cell line, SV2_GBA1−/−_,
containing nonsense mutations in both alleles of the _GBA1_ gene (Fig. 4a and Supplementary Fig. 8). This cell line fails to express GCase 1 (Fig. 4b), resulting in greatly reduced total
GCase activity (Fig. 4c). GCase 2 activity was much lower than the GCase 1 activity, and did not change as a result of _GBA1_ gene mutation (Fig. 4d). As a consequence of depletion of GCase
1, glucosylceramide, a substrate of the enzyme, accumulated in SV2_GBA1−/−_ cells (Fig. 4e). SV2_GBA1−/−_ cells were characterized by accumulation of lysosomal substrates such as p62 (Fig.
5a) and polyubiquitinated proteins (Fig. 5b), suggesting lysosomal dysfunction. Consistent with the lysosomal abnormalities, these cells had increased Lysotracker-positive structures (Fig.
5c), reduced degradation of ectopically introduced dextran (Fig. 5d) and exhibited accumulation of vacuolar structures in the cytoplasm (Fig. 5e), all of which clearly demonstrated lysosomal
impairment. In addition, we confirmed that _GBA1_ gene deletion caused lysosomal dysfunction in parental SH-SY5Y cells (data not shown). _GBA1_ DEFICIENCY POTENTIATED TRANSMISSION OF
Α-SYNUCLEIN We next examined whether _GBA1_ deletion affects cell-to-cell transmission of α-synuclein. When V1S cells were cocultured with SV2_GBA1−/−_ cells, the percentage of BiFC-positive
cells was significantly increased compared with V1S/SV2 cocultures (Fig. 6a). We interpret this to reflect the reduced capacity of SV2_GBA1−/−_ cells to clear internalized aggregates due to
lysosomal impairment (Fig. 6b). We then examined the effects of _GBA1_ gene deletion on contiguous transmission. Coculturing of V1S and SV2_GBA1−/−_ cells resulted in a significant increase
in the number of BiFC-positive cells relative to V1S/SV2 cocultures during several passages (Fig. 6c,d). Similarly, levels of α-synuclein oligomers were higher in the media of
V1S/SV2_GBA1−/−_ cocultures than in V1S/SV2 cocultures (Fig. 6e; Supplementary Fig. 9). Again, the ratio of BiFC-positive cells between V1S and SV2_GBA1−/−_ did not change significantly
during the subculture (Supplementary Fig. 10). Further indicating the role of GCase activity in this process, this phenomenon was reversed by AAV vector-mediated ectopic expression of the
wild-type _GBA1_ gene, but not in that encoding the activity-deficient E235K mutant (Fig. 6f,g). To ensure that the results of the _GBA1_ gene deletion did not represent ‘off-target’
effects, we next performed RNA interference (RNAi) experiments using AAV vectors. Reduction of GCase 1 expression using two different small hairpin RNAs (shRNAs) was confirmed with western
analysis and activity assays (Supplementary Fig. 11). Knockdown of GCase 1 production resulted in a consistent increase in cell-to-cell transfer of α-synuclein. This effect of GCase 1
knockdown was reversed by recovery of GCase 1 production, hence the recovered activity, from ectopic expression of the wild-type _GBA1_ gene (Supplementary Fig. 12). Collectively, these
results show that _GBA1_ depletion promoted perpetual transmission of α-synuclein aggregates and that ectopic expression of wild-type _GBA1_ reversed this effect, suggesting that loss of
_GBA1_ function increases α-synuclein aggregate spreading. We have previously shown that transgenic human α-synuclein was transferred from host cells to engrafted cells10. To validate the
role of _GBA1 in vivo_, we performed a transplantation experiment in which normal SH-SY5Y cells and _GBA1−/−_ (SH-_GBA1−/−_) cells were transplanted into the hippocampus of transgenic mice
expressing human α-synuclein, and transfer of α-synuclein from host cells to the grafted cells was analysed. The grafted SH-SY5Y cells do not overexpress α-synuclein, only express small
amount of endogenous α-synuclein, which hardly shows up in regular immunological detection protocols (Fig. 7a–c). We found that host-derived α-synuclein was deposited at a higher rate in
SH-_GBA1−/−_ cells than in normal SH-SY5Y cells (Fig. 7a–c). Moreover, co-immunofluorescence analysis showed that SH-SY5Y _GBA1−/−_ cells accumulated host-derived α-synuclein at higher
levels than normal SH-SY5Y cells (Fig. 7d,e). Note that although SH-SY5Y cells express low levels of endogenous α-synuclein, we failed to detect expression of endogenous α-synuclein in
engrafted SH-SY5Y cells (Fig. 7a–c). SH-SY5Y cells are human neuroblastoma cells that produce catecholamines, including dopamine. To ensure that our analysis was specific for engrafted
cells, we incorporated immunostaining for tyrosine hydroxylase (TH), and found that levels of host-derived α-synuclein were higher in TH-positive engrafted SH-SY5Y _GBA1−/−_ cells than in
TH-positive engrafted SH-SY5Y cells (Fig. 7f,g). Although there are TH-positive fibres in the hippocampus, there are no TH-positive cells. Thus, TH-positive cells in the hippocampus
represent the grafted cells. We also performed a control experiment where differentiated SH-SY5Y cells were tagged with the enhanced green fluorescence protein (eGFP) via lentiviral
infection and co-labelled for TH. We found that in _in vitro_ approximately 95% of the eGFP-positive cells were TH-positive. Likewise, about 80% of the grafted SH-SY5Y-eGFP cells in the
mouse hippocampus (_n_=5) were TH-positive (Supplementary Fig. 13). DISCUSSION The current study demonstrated that cell-to-cell transmission of α-synuclein aggregates is mediated by a
seeding mechanism and that secondary secretion of the resulting seeded aggregates mediates contiguous propagation of α-synuclein aggregates. In addition, the contiguous propagation of
aggregates is significantly promoted by lysosomal dysfunction secondary to loss of _GBA1 f_unction. In PD patients with heterozygous mutations of _GBA1_, GCase activities and protein levels
were reduced19, indicating pathological consequences of _GBA1_ heteroinsufficiency. Moreover, _GBA1_ mutation carriers have twice the number of cortical Lewy bodies than those of
noncarriers20. In addition, heterozygous mutations of _GBA1_ have been associated with increased risk of cognitive impairment21 and more rapid disease progression22, supporting the
hypothesis that the rate of spreading of Lewy pathology determines the rate of clinical progression. The current study provides evidence that α-synuclein transmission is not a single
discontinuous event, but a perpetual one. First, continuous subculture of V1S/SV2 coculture showed increased BiFC-positive cell population, instead of its decrease. This observation that
there is no dilution effect of BiFC-positive cells as the cell population grows suggests perpetual propagation of α-synuclein aggregates. Second, we found release of BiFC-positive species
from V1S/SV2 cocultures, which can only be explained by secondary release of these species after co-aggregation of transferred and resident α-synuclein in the recipient cells. This secondary
release of aggregates is a pre-requisite for perpetual propagation of aggregates. Third, ectopic introduction of V1S protein, released from V1S cells, rapidly resulted in BiFC-positive
punctates in SV2 cells, and BiFC-positive aggregate species were gradually released from SV2 cells, as cellular BiFC species gradually decreased. These results collectively support the
notion that α-synuclein aggregates perpetually propagate through the continuous cycle of exocytosis, endocytosis, seeded aggregation and secondary exocytosis. After several passage of the
coculture, the BiFC-positive population reached a steady state, the balance between aggregate production/propagation and degradation as well as cell proliferation rate, which will determine
the rate of aggregate dilution. The dilution effect owing to cell proliferation will not be a factor _in vivo_, since neurons are postmitotic. We suggest that lysosomal degradation is the
main force behind the limited increase in BiFC-positive population during successive passages. Increased steady state levels of BiFC-positive cell population with _GBA1−/−_ cells supports
this idea. Cell-to-cell transmission we assay with the new dual-cell BiFC system is a relatively infrequent event. We typically observe 2–5% cells with BiFC-positive puncta, though the
number varies depending on the culture conditions, such as cell density. The BiFC fluorescence we observe in this system is not an artefact, because either cell line alone rarely shows BiFC
signal. Neuroblastoma cells we used in this study produce catecholamines, such as dopamine, which are vulnerable to oxidation. Occasional occurrence of BiFC-positive cells in each cell line
is probably due to autofluorescence generated by oxidation of catechols. Although we used extreme caution in handling these cell lines (we only used 2-week to 2-month-old cells), some cells
generated this nonspecific background, usually in less than 0.5% of cells. Previous studies showed that α-synuclein can be transferred from neurons to astrocytes23 and oligodendrocytes24. In
our dual-cell BiFC system, V1S cells secrete α-synuclein much better than SV2 cells (Fig. 1), so we postulate that V1S cells are the predominant donor cells at least in the initial
transfer. In the current study, we intended to investigate the role of GCase 1 in the recipient cells, because the primary goal of the study was to assess the role of lysosomal function in
the clearance of transferred α-synuclein and its consequence to aggregates propagation. For this reason, we used SV2_GBA1−/−_ in this study. However, it will be important to address the role
of neuronal GCase 1 activity in neuron-to-glia transmission of α-synuclein in the future studies. Although the current study suggests that loss of _GBA1_ function has a role in
synucleinopathies, this does not necessarily rule out the pathological consequences of gain of _GBA1_ function mutations. For example, previous studies have associated mutant _GBA1_ with
impaired endoplasmic reticulum-associated degradation25 and physical interaction between α-synuclein and GCase has been demonstrated under acidic conditions26. These studies support a
potential role for gain-of-function _GBA1_ mutations, and stress the point that _GBA1_ mutations may exert their pathogenic actions via multiple mechanisms. That said, the current study
demonstrates that ectopic expression of wild-type _GBA1_, but not an activity-deficient _GBA1_ mutant, reversed the effects of _GBA1_ deletion on the propagation of α-synuclein aggregates,
presenting an attractive therapeutic opportunity for idiopathic PD. These results are in good agreement with previous studies demonstrating amelioration of synucleinopathy lesions in the
brains of the GD mouse model (D409V/D409V) by viral-mediated expression of _GBA1_ (ref. 27). Taken together, our work strongly implicates GCase as a disease-modifying therapeutic target, and
suggests that restoring the activity of this protein might retard the propagation of Lewy pathology, thereby halting the progression of PD. METHODS MATERIALS The following antibodies were
used in this study: α-synuclein monoclonal antibody (1:1,500 dilution; BD Biosciences; #610787, San Diego, CA), α-synuclein monoclonal antibody #274 (1:1,500 dilution;), phosphorylated
α-synuclein polyclonal antibody (1:100 dilution; Abcam, ab59264; Cambridge, MA), GFP (C terminus) polyclonal antibody (1:100 dilution; IMGENEX, #5127A; San Diego, CA), GFP (N terminus)
polyclonal antibody (1:500 dilution; Cell Signaling Technology, #2555; Beverly, MA), GFP (N terminus) monoclonal antibody (1:500 dilution; Abcam, ab127417), GCase monoclonal antibody 8E4
(1:1,000 dilution; from J. Barranger, University of Pittsburgh), GCase polyclonal antibody (1:1,000 dilution; Sigma, G4171; St Louis, MO), p62 monoclonal antibody (1:1,000 dilution; BD
Transduction Laboratories, #c2384-0B; Swampscott, MA), ubiquitin polyclonal antibodies (1:1,000 dilution; Dako; Glostrup, Denmark and Chemicon; Temecula, CA), and β-actin monoclonal antibody
(1:10,000 dilution; Sigma). Fluorescence dye-conjugated goat anti-rabbit IgG was purchased from Jackson Immunoresearch Laboratories (1:500 dilution; West Grove, PA). The Q tracker 595 cell
labelling kit was purchased from Invitrogen (Carlsbad, CA). CONSTRUCTION OF STABLE CELL LINES To generate stable cell lines, SH-SY5Y human neuroblastoma cells (ATCC CRL-2266; Manassas, VA)
were transfected with Venus1-αSyn (V1S) or αSyn-Venus2 (SV2) plasmid (kind gifts from Dr Pamela McLean, Massachusetts General Hospital, Charlestown, MA) using electroporation. Transfected
cells were selected with 600 μg ml−1 G418 (Invitrogen) for 2–3 weeks until colonies emerged. The stable cell lines were maintained with 200 μg ml−1 G418. GENERATION OF _GBA1_ KNOCKOUT (KO)
CELL LINES SH-SY5Y cells were transfected with plasmids encoding ZFN and a magnetic reporter (ToolGen; Seoul, Korea) using electroporation. After incubation for 48 h, cells were subjected to
magnetic separation. After trypsinization, cells were mixed with magnetic bead-conjugated antibody against H-2Kk (MACSelect Kk microbeads; Miltenyi Biotech; Germany) and the mixture was
applied to a MACS LS column (Miltenyi Biotech). Single cells obtained from the eluates were maintained until the clonal colony was picked from the culture dish. Nonsense mutations in the
_GBA1_ gene were confirmed using DNA sequencing. CELL CULTURE SH-SY5Y human neuroblastoma cell lines were as described previously28. Briefly, cells were subcultured at 37 °C in humidified
air with 5% CO2 in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum, 100 units ml−1 penicillin and 100 units ml−1 streptomycin every 2 days. For coculture, V1S and
SV2 (or SV2_GBA1−/−_) stable cells (180,000 cells each) were mixed in a coverslip and cultured for 3 days. To determine the continuous transmission of α-synuclein, the mixture of V1S and SV2
(or SV2_GBA1−/−_) cells was subcultured every 2 days (48 h). Growth rates of SV2 and SV2_GBA1−/−_ were not significantly different during the passage experiment (Supplementary Fig. 14). To
determine the effects of media washing on transmission, the V1S/SV2 coculture was washed with DMEM and incubated with fresh growth media the day before assay. To determine the effects of
antibodies on the transmission of α-synuclein, 5 μg ml−1 of control IgG or Ab274 was added to V1S/SV2 coculture the day before the assay. PREPARATION OF Α-SYNUCLEIN CONDITIONED MEDIA The
α-synuclein conditioned media were obtained from 20 dishes of 100-mm dish. When V1S cells are 90% confluent, the medium were replaced with serum-free DMEM after washing three times with
DMEM. Cells were incubated at 37 °C for 18 h. Conditioned media (100 ml) were collected from 20 dishes of V1S cells. After centrifugation at 1,000 _g_ for 10 min, supernatant was centrifuged
at 10,000 for 20 min to remove the cell debris. The supernatant was concentrated to 200-fold using Amicon 10K MWKO filter (Millipore, Billerica, MA). SIZE-EXCLUSION CHROMATOGRAPHY
Size-exclusion chromatography was performed using AKTA purifier (GE Healthcare Life Science, Piscataway, NJ). Samples were applied to Superdex 200 HR 10/30 column (GE Healthcare Life
Science) equilibrated with phosphate buffer (20 mM sodium phosphate, pH 7.4, 0.15 M NaCl) and eluted at a flow rate of 0.5 ml per minute. GCASE ACTIVITY AND GLYCOSPHINGOLIPID ASSAY Cellular
GCase activity was determined as described previously27. Briefly, cells were analysed for GCase activity using 10 mM 4-methylumbelliferyl-β-D-glucoside (Sigma) in buffer containing 1% bovine
serum albumin at 37 °C for 1 h. After adding 0.5 volume of 1 M glycine buffer, pH 12.5, cleaved 4-methylumbelliferone was measured using Spectramax Gemini fluorimeter (excitation at 365 nm,
emission at 445 nm; Molecular Devices, Sunnyvale, CA). All measurements were done without taurocholate, a detergent that activates the GCase enzymatic activity. GCase2 specific activity was
determined in the presence of the GCase 1 inhibitor, conduritol-B-epoxide (100 μM). GCase 1 activity was obtained by subtracting the GCase2 activity levels from the total GCase activity.
Cellular GlcCer and GalCer levels were measured by mass spectrometry as previously described27. Briefly, organic cellular extracts were injected onto an Atlantis HILIC silica column (Waters
Corp.; Milford, MA) for separation of GlcCer and GalCer, which were detected using an AB Sciex API-5000 mass spectrometer. INFECTION WITH ADENO-ASSOCIATED VIRUS (AAV) VECTORS After passage,
cells were co-infected with Ad-TS129 (3 M.O.I.) and various AAV (5e6 M.O.I.). Cells were incubated at 39 °C for 24 h for activation of the temperature-sensitive helper adenovirus. Several
AAV vectors were designed for knockdown of _GBA1_ (_GFP_-miRNA _GBA1a_ and _GBA1b_) and a rescue vector expressing a miRNA-resistant _GBA1_* (_GBA1_*-miRNA _GBA1b_). QUANTIFICATION OF
SECRETED Α-SYNUCLEIN AGGREGATES To measure the level of secreted α-synuclein co-aggregates, culture medium obtained from V1S/SV2 coculture was centrifuged at 10,000 _g_ for 10 min. The
supernatant from culture media was transferred to a 96-well black plate (Corning Inc.; Corning, NY) and subjected to fluorometric analysis using a fluorescence microplate reader (SpectraMax
Gemini EM;, Molecular Devices, Sunnyvale, CA). The procedure for the ELISA was performed as described previously29. Briefly, 1 μg ml−1 of the capture antibody #62 in 50 mM carbonate buffer
(pH 9.6) was coated on a 96-well ELISA plate (Maxisorp, Nunc; Rochester, NY) overnight at 4°C. After washing with phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBST), SuperBlock
T20 PBS blocking buffer (Pierce; Rockford, IL) was added for 1 h at room temperature (RT) with shaking. After washing with PBST, α-synuclein aggregates from the standard and culture media
were incubated at RT for 2.5 h with shaking. Plates were washed again with PBST, after which 1 μg ml−1 of biotinylated reporter antibody #62 was added and incubated at RT for 1.5 h. After
washing with PBST, avidin-conjugated peroxidase (ExtrAvidin; Sigma; St Louis, MO) was added to the plate. The plate was then incubated with 3,3′5,5′-tetramethylbenzidine solution (Sigma).
After addition of 2 N H2SO4, absorbance was measured at 490 nm using a SpectraMax 190 spectrophotometer (Molecular Devices). PREPARATION OF CELL EXTRACTS After washing with ice-cold PBS,
cells were lysed in extraction buffer (1% Triton X-100, 1% (v/v) protease inhibitor cocktail (Sigma) in PBS). Cell lysates were incubated on ice for 10 min and centrifuged at 16,000 _g_ for
10 min. The Triton X-100 insoluble fraction was resuspended in 1X Laemmli sample buffer and sonicated briefly. WESTERN BLOTTING Western blotting was performed as previously described30.
Images were obtained and quantified using the FUJIFILM Luminescent Image Analyser LAS-3000 and Multi Gauge (v3.0) software (FUJIFILM; Tokyo, Japan). IMMUNOFLUORESCENCE STAINING The procedure
for immunofluorescence staining was performed as previously described30. Briefly, cells grown on poly-L-Lysine-coated coverslips were fixed in 4% paraformaldehyde in PBS and permeabilized
in 0.1% Triton X-100 in PBS. After incubation in blocking solution (5% bovine serum albumin/3% goat serum in PBS), primary antibodies diluted in blocking solution were added to the cells.
After washing, cells were incubated with fluorescent dye-conjugated secondary antibodies. Nuclei were stained with TOPRO-3 iodide (Invitrogen). Cells were mounted onto slide glasses in the
presence of Prolong Gold Antifade Reagent (Invitrogen). Olympus FV1000 confocal laser scanning microscopy was used for observation of cells. CHARACTERIZATION OF LYSOSOMAL DYSFUNCTION For
imaging of the lysotracker-positive compartment, SH-SY5Y cells were stained with 75 nM lysotracker solution in dimethyl sulphoxide (Lysotracker Red DND-99; Invitrogen) diluted in growth
media, and incubated for 1 h at 37 °C in a CO2 incubator. After washing with ice-cold PBS, cells were fixed in a 4% paraformaldehyde (PFA) solution. To determine the degradation ratio of
internalized dextran, cells were incubated with 20 μg ml−1 of fluorescein isothiocyanate-labelled dextran (Invitrogen) for 2 h. After washing with DMEM, cells were incubated with fresh
growth media for 30 min and fixed with a 4% PFA solution. The fluorescence intensity was measured using Olympus FV1000 software. ELECTRON MICROSCOPY Cells were grown in 100-mm dishes and
fixed in the Karnovsky’s fixative solution (2% glutaraldehyde, 2% paraformaldehyde, 0.5% CaCl2). After immersing in 1% osmium tetraoxide for 1.5 h, cells were dehydrated with 50, 60, 70, 80,
90, 95 and 100% of absolute ethanol. Cells were infiltrated with propylene oxide and EPON mixture (EPON 812, MNA, DDSA, DMP30) for 10 min before embedding EPON mixture. After embedding, the
cells were sectioned with LEICA EM UC-7 Ultra-microtome (Leica Microsystems, Austria), then stained with 6% uranyl acetate and lead citrate. The grids were observed using transmission
electron microscopy JEM-1011 (JEOL; Japan) and analysed using Megaview III software (Soft imaging system, Germany). For morphometric analysis, 15 cells were analysed for each experiment.
UPTAKE OF EXTRACELLULAR Α−SYNUCLEIN FIBRILS Cells were incubated with 0.2 μM of sonicated α-synuclein fibrils for 1 day and fixed with 4% PFA. After immunofluorescence staining, the
intensity of α-synuclein in a single cell was measured using Olympus FV1000 software. ANIMALS For this study we used heterozygous transgenic mice (Line 61) expressing wild-type human
α-synuclein under the control of the mThy1 promoter31. These mice were selected since they display extensive neuronal and synaptic accumulation of α-synuclein aggregates throughout the
neocortex, limbic system and striato-nigral system, accompanied by motor and nonmotor deficits32 similar to those observed in patients with PD and dementia with Lewy bodies. Moreover, we
have previously shown that α-synuclein in these mice is transmitted from neurons to grafted cells10, permitting the evaluation of the effects of _GBA1_ on transmission. All the procedures
for animal experiments were approved by the Institutional Animal Care and Use Committee. STEREOTAXIC DELIVERY OF _GBA1−/−_ CELLS α-Synuclein transgenic mice and their non-transgenic litter
mates (_n_=8 per group, 10-month-old, total four groups, 32 mice) received unilateral stereotaxic injections of a 2 μl suspension of wild-type or _GBA1−/−_ cell preparation (1.2 million
cells) into the hippocampus as previously described10. Mice were anaesthetized and placed on a Koft stereotaxic apparatus. Utilizing an electronic delivery pump system, SH-SY5Y or SH-SY5Y
_GBA−/−_ cell preparations were injected using a Hamilton syringe. Coordinates for the hippocampus were as follows: AP −2.0 mm, lateral 1.5 mm, depth 1.3 mm. Mice survived for 4 weeks after
the graft injection. Following NIH guidelines for the humane treatment of animals, mice were anaesthetized with chloral hydrate and flush-perfused transcardially with 0.9% saline. Brains
were removed and fixed in phosphate-buffered 4% PFA (pH 7.4) at 4 °C for 48 h for neuropathological analysis. IMMUNOCYTOCHEMICAL ANALYSIS AND CONFOCAL MICROSCOPY Brains were serially
sectioned at 40 μm using a vibratome (Leica; Deerfield, IL, USA). Serial, free-floating, blind-coded vibratome sections from transgenic and non-transgenic mice grafted with WT and SH-SY5Y
_GBA1−/−_ cells were immunostained as previously described33 with antibodies against total α-synuclein (Millipore), α-synuclein C terminus (SYN105 antibody), and human α-synuclein (SYN211).
Sections were then incubated with biotin-tagged secondary antibodies and developed with diaminobenzidine. Sections immunolabeled with antibodies against α-synuclein (three from each mouse at
100-μm intervals) were analysed via the dissector method using the Stereo-Investigator System (MBF Bioscience; Williston, VT) and the results were averaged and expressed as the percentage
of positive cells in the grafted area. To determine the colocalization between α-synuclein and neuronal markers, double-labelling experiments were performed, as previously described34. For
this purpose, vibratome sections were immunolabeled using an antibody against human α-synuclein (SYN211) and antibodies against TH (Millipore) and GCase (Abcam, ab55080). The TH- and
GCase-immunoreactive grafted cells were detected with fluorescein isothiocyanate-tagged antibodies (1:75; Vector; Burlingame, CA), while α-synuclein was detected with Tyramide Red (NEN Life
Sciences). All sections were processed simultaneously under the same conditions, and experiments were performed in duplicate to assess the reproducibility of results. Sections were imaged
with a Zeiss 63X (N.A. 1.4) objective on an Axiovert 35 microscope (Zeiss) with an attached MRC1024 laser scanning confocal microscope (LSCM) system (BioRad)34. Series of paired optical
sections were analysed with ImageJ colocalization colour map software to determine the α-synuclein pixel intensity associated with WT and _GBA1−/−_ cells. An average of 20 digital images was
analysed per mouse. Each digital image contained an average of four cells. Values in the figures are expressed as means±s.e.m. STATISTICAL ANALYSIS Values shown in the figures are presented
as mean±s.e.m. To determine the statistical significance, _P_ values were calculated by means of paired, two-tailed Student’s _t_-tests using GraphPad InStat version 3.05 software.
ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Bae, E.-J. _et al_. Glucocerebrosidase depletion enhances cell-to-cell transmission of α-synuclein. _Nat. Commun._ 5:4755 doi:
10.1038/ncomms5755 (2014). REFERENCES * Jellinger, K. A. Alpha-synuclein pathology in Parkinson's and Alzheimer's disease brain: incidence and topographic distribution—a pilot
study. _Acta Neuropathol._ 106, 191–201 (2003). Article PubMed Google Scholar * Brundin, P., Melki, R. & Kopito, R. Prion-like transmission of protein aggregates in neurodegenerative
diseases. _Nat. Rev. Mol. Cell. Biol._ 11, 301–307 (2010). Article CAS PubMed PubMed Central Google Scholar * Lee, S. J., Desplats, P., Sigurdson, C., Tsigelny, I. & Masliah, E.
Cell-to-cell transmission of non-prion protein aggregates. _Nat. Rev. Neurol._ 6, 702–706 (2010). Article PubMed PubMed Central Google Scholar * Angot, E. et al. Alpha-synuclein
cell-to-cell transfer and seeding in grafted dopaminergic neurons _in vivo_. _PLoS ONE_ 7, e39465 (2012). Article ADS CAS PubMed PubMed Central Google Scholar * Danzer, K. M. et al.
Exosomal cell-to-cell transmission of alpha synuclein oligomers. _Mol. Neurodegener._ 7, 42 (2012). Article CAS PubMed PubMed Central Google Scholar * Lee, H. J., Bae, E. J. & Lee,
S. J. Extracellular alpha-synuclein-a novel and crucial factor in Lewy body diseases. _Nat. Rev. Neurol._ 10, 92–98 (2014). Article CAS PubMed Google Scholar * Pan, T., Kondo, S., Le, W.
& Jankovic, J. The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson's disease. _Brain_ 131, (Pt 8): 1969–1978 (2008). Article PubMed Google
Scholar * Sidransky, E. et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. _N. Engl. J. Med._ 361, 1651–1661 (2009). Article CAS PubMed PubMed
Central Google Scholar * Nalls, M. A. et al. A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. _JAMA Neurol._ 70, 727–735 (2013). Article PubMed Google
Scholar * Desplats, P. et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. _Proc. Natl Acad. Sci. USA_ 106, 13010–13015 (2009).
Article ADS CAS PubMed Google Scholar * Lee, H.-J. et al. Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. _Int. J. Biochem. Cell Biol._ 40, 1835–1849
(2008). Article CAS PubMed Google Scholar * Goncalves, S. A., Matos, J. E. & Outeiro, T. F. Zooming into protein oligomerization in neurodegeneration using BiFC. _Trends Biochem.
Sci._ 35, 643–651 (2010). Article CAS PubMed Google Scholar * Outeiro, T. F. et al. Formation of toxic oligomeric alpha-synuclein species in living cells. _PLoS ONE_ 3, e1867 (2008).
Article ADS PubMed PubMed Central Google Scholar * Fujiwara, H. et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. _Nat. Cell. Biol._ 4, 160–164 (2002). Article CAS
PubMed Google Scholar * Spillantini, M. G., Crowther, R. A., Jakes, R., Hasegawa, M. & Goedert, M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's
disease and dementia with lewy bodies. _Proc. Natl Acad. Sci. USA_ 95, 6469–6473 (1998). Article ADS CAS PubMed Google Scholar * Kahle, P. J. alpha-Synucleinopathy models and human
neuropathology: similarities and differences. _Acta Neuropathol._ 115, 87–95 (2008). Article CAS PubMed Google Scholar * Kim, C. et al. Neuron-released oligomeric alpha-synuclein is an
endogenous agonist of TLR2 for paracrine activation of microglia. _Nat. Commun._ 4, 1562 (2013). Article PubMed PubMed Central Google Scholar * Jang, A. et al. Non-classical exocytosis
of alpha-synuclein is sensitive to folding states and promoted under stress conditions. _J. Neurochem._ 113, 1263–1274 (2010). CAS PubMed Google Scholar * Gegg, M. E. et al.
Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. _Ann. Neurol._ 72, 455–463 (2012). Article CAS PubMed PubMed Central Google Scholar * Clark, L. N. et al.
Association of glucocerebrosidase mutations with dementia with lewy bodies. _Arch. Neurol._ 66, 578–583 (2009). Article PubMed PubMed Central Google Scholar * Alcalay, R. N. et al.
Cognitive performance of GBA mutation carriers with early-onset PD: the CORE-PD study. _Neurology_ 78, 1434–1440 (2012). Article CAS PubMed PubMed Central Google Scholar *
Winder-Rhodes, S. E. et al. Glucocerebrosidase mutations influence the natural history of Parkinson's disease in a community-based incident cohort. _Brain_ 136, 392–399 (2013). Article
PubMed Google Scholar * Lee, H. J. et al. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. _J. Biol. Chem._ 285, 9262–9272
(2010). Article CAS PubMed PubMed Central Google Scholar * Reyes, J. F. et al. Alpha-synuclein transfers from neurons to oligodendrocytes. _Glia_ 62, 387–398 (2014). Article PubMed
Google Scholar * Ron, I. & Horowitz, M. ER retention and degradation as the molecular basis underlying Gaucher disease heterogeneity. _Hum. Mol. Genet._ 14, 2387–2398 (2005). Article
CAS PubMed Google Scholar * Yap, T. L. et al. Alpha-synuclein interacts with Glucocerebrosidase providing a molecular link between Parkinson and Gaucher diseases. _J. Biol. Chem._ 286,
28080–28088 (2011). Article CAS PubMed PubMed Central Google Scholar * Sardi, S. P. et al. CNS expression of glucocerebrosidase corrects alpha-synuclein pathology and memory in a mouse
model of Gaucher-related synucleinopathy. _Proc. Natl Acad. Sci. USA_ 108, 12101–12106 (2011). Article ADS CAS PubMed Google Scholar * Lee, H.-J., Khoshaghideh, F., Patel, S. & Lee,
S.-J. Clearance of alpha-synuclein oligomeric intermediates via the lysosomal degradation pathway. _J. Neurosci._ 24, 1888–1896 (2004). Article CAS PubMed Google Scholar * Lee, H. J. et
al. Enzyme-linked immunosorbent assays for alpha-synuclein with species and multimeric state specificities. _J. Neurosci. Methods_ 199, 249–257 (2011). Article CAS PubMed Google Scholar
* Lee, H.-J. & Lee, S.-J. Characterization of cytoplasmic alpha-synuclein aggregates. fibril formation is tightly linked to the inclusion-forming process in cells. _J. Biol. Chem._
277, 48976–48983 (2002). Article CAS PubMed Google Scholar * Rockenstein, E. et al. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the
platelet-derived growth factor and Thy-1 promoters. _J. Neurosci. Res._ 68, 568–578 (2002). Article CAS PubMed Google Scholar * Fleming, S. M. et al. Early and progressive sensorimotor
anomalies in mice overexpressing wild-type human alpha-synuclein. _J. Neurosci._ 24, 9434–9440 (2004). Article CAS PubMed Google Scholar * Bae, E. J. et al. Antibody-aided clearance of
extracellular alpha-synuclein prevents cell-to-cell aggregate transmission. _J. Neurosci._ 32, 13454–13469 (2012). Article CAS PubMed PubMed Central Google Scholar * Masliah, E. et al.
Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. _PLoS ONE_ 6, e19338 (2011). Article ADS CAS PubMed
PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the National Research Foundation (NRF) grant funded by the Korean Government (to S.-J.L.;
2010-0015188), the Korea Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (to S.-J.L.; A111228), and by the NIH (to E.M.; AG18440, AG022074). This paper
was written as part of Konkuk University's research support program for its faculty on sabbatical leave in 2013. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Biomedical
Science and Technology, Konkuk University, Seoul, 143-701, Korea Eun-Jin Bae, Na-Young Yang, Miyoung Song, Jun Sung Lee, Byung Chul Jung & Seung-Jae Lee * Department of Anatomy, School
of Medicine, Konkuk University, Seoul, 143-701, Korea Cheol Soon Lee & He-Jin Lee * Department of Biomedical Laboratory Science, College of Health Science, Yonsei University, Wonju,
220-710, Korea Byung Chul Jung * ToolGen, Inc., Biotechnology Incubating Center, Seoul National University, Seoul, 305-390, Korea Seokjoong Kim * Departments of Pathology and Neurosciences,
University of California, San Diego, La Jolla, 92093-0624, California, USA Eliezer Masliah * Genzyme, a Sanofi Company, Framingham, 01701, Massachusetts, USA Sergio Pablo Sardi * College of
Veterinary Medicine, Konkuk University, Seoul, 143-701, Korea Seung-Jae Lee Authors * Eun-Jin Bae View author publications You can also search for this author inPubMed Google Scholar *
Na-Young Yang View author publications You can also search for this author inPubMed Google Scholar * Miyoung Song View author publications You can also search for this author inPubMed Google
Scholar * Cheol Soon Lee View author publications You can also search for this author inPubMed Google Scholar * Jun Sung Lee View author publications You can also search for this author
inPubMed Google Scholar * Byung Chul Jung View author publications You can also search for this author inPubMed Google Scholar * He-Jin Lee View author publications You can also search for
this author inPubMed Google Scholar * Seokjoong Kim View author publications You can also search for this author inPubMed Google Scholar * Eliezer Masliah View author publications You can
also search for this author inPubMed Google Scholar * Sergio Pablo Sardi View author publications You can also search for this author inPubMed Google Scholar * Seung-Jae Lee View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS E.-J.B. designed and performed the majority of experiments, analysed the data and was involved in the
manuscript preparation. M.S. and H.-J.L. designed and generated the V1S and SV2 cell lines and characterized the dual-cell BiFC model. N.-Y.Y. and C.S.L. performed the experiments with
E.-J.B. J.S.L. and B.C.J. performed the size-exclusion chromatography. S.J.K. designed and constructed zinc-finger nuclease plasmids. E.M. designed, performed and analysed the mouse
transplantation experiments. S.P.S. conceived the study, provided the AAVs, performed GCase activity assays and lipid analysis, and was involved in manuscript editing. S.-J.L. conceived and
coordinated the study, designed experiments, analysed the data and wrote the paper. CORRESPONDING AUTHOR Correspondence to Seung-Jae Lee. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figures 1-14 (PDF 1574 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Bae, EJ., Yang, NY., Song, M. _et al._ Glucocerebrosidase depletion enhances cell-to-cell transmission of α-synuclein. _Nat Commun_ 5, 4755 (2014).
https://doi.org/10.1038/ncomms5755 Download citation * Received: 22 February 2014 * Accepted: 18 July 2014 * Published: 26 August 2014 * DOI: https://doi.org/10.1038/ncomms5755 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative

:max_bytes(150000):strip_icc():focal(216x0:218x2)/benedict-cumberbatch-1-435-4-20cc736017b24435a3498a49d7c22b0e.jpg)
:max_bytes(150000):strip_icc():focal(584x489:586x491)/bill-gates-01-122122-3dca1a10ca5b4473af97402696129443.jpg)