- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The greatest potential environmental benefit of metabolic engineering would be the production of high-volume commodity chemicals, such as biofuels. Yet, the high yields required for
the economic viability of low-value chemicals is particularly hard to achieve in microbes owing to the myriad competing biochemical pathways. An alternative approach, which we call
synthetic biochemistry, is to eliminate the organism by constructing biochemical pathways _in vitro_. Viable synthetic biochemistry, however, will require simple methods to replace the
cellular circuitry that maintains cofactor balance. Here we design a simple purge valve module for maintaining NADP+/NADPH balance. We test the purge valve in the production of
polyhydroxybutyryl bioplastic and isoprene—pathways where cofactor generation and utilization are unbalanced. We find that the regulatory system is highly robust to variations in cofactor
levels and readily transportable. The molecular purge valve provides a step towards developing continuously operating, sustainable synthetic biochemistry systems. You have full access to
this article via your institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS DESIGN OF MICROBIAL CATALYSTS FOR TWO-STAGE PROCESSES Article 22 August 2024 ENGINEERED AUTONOMOUS
DYNAMIC REGULATION OF METABOLIC FLUX Article 21 December 2023 GENOME-SCALE METABOLIC REWIRING IMPROVES TITERS RATES AND YIELDS OF THE NON-NATIVE PRODUCT INDIGOIDINE AT SCALE Article Open
access 23 October 2020 INTRODUCTION Metabolic engineering and synthetic biology have been employed for the production of high-value chemicals but has not been as successful in meeting the
stringent economics of large-scale commodity chemical manufacturing1. Microbial systems are often hampered by a variety of technical challenges that make it hard to achieve cost
competitiveness, including poor yields owing to competing pathways; low productivity caused by slow growth rates or difficulties in pathway optimization; contaminating microbial growth;
product toxicity; and expensive product isolation2,3. Another approach that is beginning to receive attention is to perform complex biochemical transformations using mixtures of enzymes in a
reaction vessel rather than within a cell. Building single, dedicated pathways _in vitro_ can eliminate side reactions that occur in the cell, so that nearly 100% yields and fast reaction
times are possible3,4,5. _In vitro_ biochemical systems also allow for more precise control over optimization and product toxicity problems can be more easily diagnosed and mitigated6,7.
Moreover, product extraction can be more facile. Traditionally, _in vitro_ pathway construction has been relegated to use as a research tool or in applications that require only 1—3 enzymes
for the production of chiral compounds and other high-value chemicals1,8,9. Improvements in protein expression and access to stable enzymes have made more complex systems possible,
however1,9,10,11,12. _In vitro_ biotransformation systems have been reported in recent years involving systems of over 30 enzymes13,14. One of the first modern studies in this area was an
artificial pathway that produced hydrogen from starch1,15,16,17. The concept was recently advanced with a creative system that generated hydrogen from cellobiose at nearly 100% yields16. In
another effort, hyper-thermophilic glycolysis enzymes were heterologously expressed, heat purified and assembled to convert glucose to _n_-butanol in 82% yield18. In another study, an
elegantly simplified non-phosphorylative Entner–Doudoroff pathway from hyper-thermophilic archaea was constructed to produce ethanol and isobutanol in ~55% yields19. These pioneering studies
illustrate the flexibility of synthetic biochemistry and the potential for high yields. A limiting feature of earlier work in synthetic biochemistry systems is the need to either feed the
systems with high-energy cofactors, or if they are generated by the pathway _in situ_, balance their use to ensure regeneration. For example, in the Sieber pathway to generate ethanol and
isopropanol from glucose19, the pathway was beautifully designed to reduce only 2 mol of NAD+ to NADH during the catabolic phase and to reoxidize 2 mol of NADH in the anabolic phase. Thus,
the utilization of NAD+ was perfectly balanced allowing multiple passes through the pathway. In other systems, the pathways were also designed to maintain cofactor balance18. The requirement
for perfect balance limits synthetic biochemistry in two ways, however. First, it places stringent constraints on pathway design, potentially limiting the types of chemicals that can be
made. Second, it may ultimately limit the number of cycles the system can perform without adding additional, expensive high-energy cofactors. Why? If there is any spontaneous oxidation of
NADH, it effectively creates an unintended cofactor imbalance because more NADH is required than would be expected based on the stoichiometry of the system. Thus, over time the levels of
NADH will dissipate and the system will wind down. As the economic viability of a synthetic biochemistry system will likely depend on the ability to run the systems in a self-sustaining
manner for long periods of time, we need to develop methods that can maintain high-energy cofactor levels without requiring perfect pathway stoichiometry. Ideally, we believe that a
synthetic biochemistry system should be able to generate more high-energy cofactors in the catabolic phase of the pathway, than is needed in the anabolic phase of the pathway so that gradual
losses by spontaneous ATP hydrolysis or NAD(P)H oxidation can be tolerated. On the other hand, if an excess of high-energy cofactors is produced, it is necessary to also find a way to
dissipate excess production so that the reactions can proceed (that is, carbon flux can be maintained). In short, we need a way to maintain cofactor balance. If it were possible to regulate
cofactor balance without affecting carbon flux, it would also allow for much more flexibility in pathway design. As a step towards this goal, we describe a synthetic biochemistry purge valve
module to maintain the proper balance of NADPH and test whether this general approach can be applied for the production of the bioplastic polyhydroxybutyrate (PHB) and isoprene. PHB and
other polyhydroxyalkanoates (PHAs) are biodegradable thermoplastics. PHAs can have characteristics similar to many popular petrochemical-derived polymers, but are nontoxic and biodegradable,
so they are attracting increased attention as a possible green alternative to petroleum-based polymers in a wide range of applications20,21,22. The best characterized and most abundant PHA
polymer is PHB that is naturally produced from acetyl-CoA as a carbon and energy storage mechanism in many organisms22. Currently, industrial production of PHB is done by _in vivo_ batch
culture processes under nutrient starvation. This process is typically very time consuming, requires large fermentation volumes and requires expensive methods for the extraction of PHB20.
Prior attempts to produce bioplastic _in vitro_ have required the addition of sacrificial substrates and a molar excess of cofactors to convert acetate to PHB23. Isoprene is a platform
chemical for a variety of products, but it is mostly employed in the production of synthetic rubber24,25,26. The isoprenoid pathway also provides precursors for over 25,000 known
biomolecules including drugs such as taxol and potential biofuels27. There have been a number of efforts to produce isoprene in microorganisms24,26,28,29 and the best reported yield is 28%
from glucose24. We recently showed that a synthetic biochemistry system could produce isoprene in >95% yield from pyruvate as long as high-energy cofactors were added30. Here we employ a
purge valve system in a pathway to convert pyruvate into PHB that maintains sustainable reducing cofactor balance, without the requirement for perfect stoichiometric matching of cofactor
generation and carbon usage. Further, we show that our purge valve module can be used as the basis for the production of other acetyl-CoA-derived products by applying it to the production of
isoprene from pyruvate via the mevalonate pathway. Regulatory modules such as this can free us from having to perfectly balance cofactor utilization when designing synthetic biochemistry
systems. RESULTS OVERVIEW AND DESIGN OF PATHWAY The biotransformation of pyruvate into PHB illustrates a basic cofactor imbalance problem that is encountered in biochemical systems. In
particular, conversion of pyruvate to acetyl-CoA by pyruvate dehydrogenase (PDH) yields one molecule of NADH. However, the three-enzyme pathway (phaA, B and C) to PHB from acetyl-CoA
utilizes only one half of a molecule of NADPH per acetyl-CoA. Thus, the canonical pathway produces an excess of reducing equivalents. Moreover, the reducing equivalents are of the wrong type
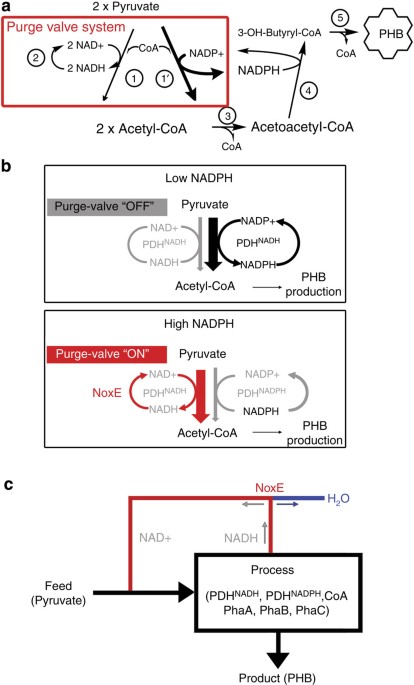
(NADH rather than NADPH). We therefore designed a pathway, shown in Fig. 1a that can generate the correct cofactor and regulate its production. In our design, we create a synthetic
biochemistry ‘purge valve’ that effectively decouples the stoichometric production of NAD(P)H from acetyl-CoA (Fig. 1). To this end, we employ a mixture of both an NAD+-utilizing wild-type
(WT) PDH (PDHNADH), a mutant PDH that utilizes NADP+ (PDHNADPH), and a water-generating NADH oxidase (NoxE) that specifically oxidizes NADH, but not NADPH31,32. By employing this metabolic
node, we generate NADPH needed for PHB production from pyruvate, but also dissipate excess reducing equivalents in an autoregulatory manner. As illustrated in Fig. 1b, under low-NADPH,
high-NADP+ conditions, the mutant PDHNADPH can operate to generate acetyl-CoA and restore NADPH levels. Under high-NADPH, low-NADP+ conditions, the PDHNADPH activity will automatically be
choked off, and the WT PDHNADH will be used preferentially to produce acetyl-CoA and NADH. In this high-NADPH condition, the reducing equivalents are not needed. Because the reducing
equivalents are produced in the form of NADH and not NADPH, they are eliminated by the oxidase, NoxE. The presence of NoxE ensures that NADH never builds up and the PDHNADH can always
operate to generate carbon for the PHB pathway in the form of acetyl-CoA. The PDHNADH/PDHNADPH/NoxE system acts like a purge valve that opens under conditions of high NADPH to relieve the
excess reducing equivalent ‘pressure’ (that is, buildup of NADH) and allow carbon flux to be maintained. An engineering schematic of the purge valve system is shown in Fig. 1c. ENZYME
ENGINEERING AND COFACTOR SPECIFICITY To implement our purge valve module, we needed an NADP+-utilizing PDH. A mutant of _Escherichia coli_ PDH has been engineered to have NADP+ specificity
by introducing mutations into the E3 enzyme (EcE3)33. The _E. coli_ PDH proved too unstable for our system, however. We therefore engineered a mutant of the thermophilic _Geobacillus
stearothermophilus_ PDH that preferentially accepts NADP+ with increased enzyme stability. Similar to the design of the _E. coli_ PDH mutant, the _G. stearothermophilus_ PDH mutant was
designed by overlaying the known structure of the _G. stearothermophilus_ E3 subunit (GsE3)33 with the known structure of the related _E. coli_ glutathione reductase, which utilizes NADP+
(ref. 33) (REF or PDBID:1GET). The structural superposition allowed us to position the additional phosphate moiety in the active site of the GsE3, based on how it was placed in glutathione
reductase (see Fig. 2). We could then design side-chain substitutions in GsE3 that might allow acceptance of the phosphate. We were guided by the prior successful design of the EcE3 enzyme,
which shares 47% sequence identity with the GsE3 (refs 33, 34). The mutations introduced into EcE3 were E206V, G207R, A208K, G209H and S213R (GsE3 numbering). After examining the changes in
the context of the GsE3 structure, we decided to introduce all but G209H, because it appeared that the new His side chain might create steric clashes with nearby K224 and N237 residues. The
kinetic properties of the engineered and WT enzymes reveal that the mutations alter specificity as desired. The kinetic parameters are listed in Supplementary Table 1. For the WT _G.
stearothermophilus_ enzyme (GsPDHNADH), _k_cat is 11.2 times higher with NAD+ than NADP+ and _k_cat/_K_m is 1,150 times higher. For the engineered mutant (GsPDHNADPH), _k_cat is 7.3 times
higher with NADP+ than NAD+ and _k_cat/_K_m is 21 times higher. Thus, we were able to flip the specificity of the PDH enzyme. The GsPDHNADH and GsPDHNADPH enzymes (henceforth designated
PDHNADH and PDHNADPH) were much more stable than their _E. coli_ counterparts. As shown in Supplementary Fig. 1, the _G stearothermophilus_ enzymes retained ~50% activity after 1 h
incubation at 67 °C, whereas the _E. coli_ PDH enzymes were completely inactivated at 50 °C. A second key requirement of the purge valve design is the use of an NADH oxidase with high
cofactor specificity. We chose NoxE from _Lactococcus lactis_ as it is a water-forming NADH oxidase so it does not generate any toxic products such as hydrogen peroxide31,32,35. As shown in
Table 1, the _K_cat of NoxE is 248.8 times greater with NADH than NADPH and _k_cat/_K_m is 9,900 times greater. ENZYME CATALYSED PRODUCTION OF PHB FROM PYRUVATE The enzymes chosen for use in
this work are listed in Table 1. In the initial tests, we only employed the WT, PDHNADH complex and NoxE to generate acetyl-CoA and supplied NADPH exogenously. After optimizing enzyme
ratios in this simple system, we then added the mutant PDHNADPH to test _in situ_ generation of NADPH. Finally, the amount of PDHNADPH was optimized, keeping the other enzymes fixed. The
progress of the optimized pyruvate to PHB reaction is shown in Fig. 3 along with a control lacking the last enzyme, phaC. Both reactions had a PDHNADPH:PDHNADH ratio of 40:1. At this ratio,
the NADPH levels rise rapidly (A340) and are maintained throughout the time course (NoxE rapidly oxidizes NADH so changes in A340 reflect only changes in NADPH levels). At the same time, PHB
granules are produced as monitored by A600 (ref. 36). We assayed the PHB production using a gas chromatography method and found that the optimized reaction produced 2.45±0.5 mg ml−1 of PHB
from 50 mM pyruvate, which represents nearly complete conversion (94±20%) of pyruvate to plastic. In the optimized system, we started with 0.5 mM NADP+, so achieving 94% yield requires over
90 turnovers of the NADP+ cofactor, indicating a high level of system sustainability. The stability of the full system was assessed by mixing components together and then initiating the
reaction at various time delays. The decrease in extent of the reaction is shown in Supplementary Fig. 2. We find that the extent of reaction remains ~50% after 2 days. AUTOREGULATORY
BEHAVIOUR OF PURGE VALVE The regulatory behaviour of the purge valve is better seen at sub-optimal enzyme concentrations and ratios of PDHNADPH to PDHNADH that slow down the response time.
In the optimized assay (40:1 mol ratio of PDHNADPH:PDHNADH), we observed a rapid rise in NADPH levels, which was sustained throughout. In the non-optimal systems shown in Fig. 4, the purge
valve cannot respond as rapidly to drops in NADPH concentrations so we can observe variations in NADPH levels as the system develops. We still observe a rapid initial rise in NADPH levels,
but as intermediates build up, the consumption starts to outstrip NADPH production. Eventually, the system compensates by generating higher levels of NADPH. The restoration of NADPH levels
would be impossible without the proper operation of the purge valve system. THE SYSTEM IS ROBUST TO COFACTOR LEVELS To test whether the system was robust to changes in cofactor levels, we
varied the initial cofactor concentrations in the reactions and measured the yields of PHB. Each reaction was constructed with combinations of NAD+, NADH, NADP+ or NADPH at either 0.1 or 1
mM and the production of PHB was monitored by the final OD at 600 nm. All of the reaction conditions were compared with the optimized reaction that produced nearly complete conversion of
pyruvate to PHB and, as shown in Fig. 5, were within random variation (see also Supplementary Table 3). This result indicates that the purge valve can compensate readily for changes in
cofactor concentrations and reduction states. PORTING THE PURGE VALVE SYSTEM TO ISOPRENE PRODUCTION To test the versatility of the molecular purge valve and whether it can be applied as a
general platform for the production of a diverse array of acetyl-CoA-derived compounds, we used the PDH purge valve to produce isoprene via the acetyl-CoA-dependent mevalonate pathway. We
have previously described the _in vitro_ production of isoprene from pyruvate, which required the use of exogenously added NADPH30. Similar to the PHB pathway, the mevalonate pathway has an
inherently different carbon and cofactor stoichiometry. In particular, the mevalonate pathway requires three acetyl-CoA and two NADPH for the production of isoprene (see Fig. 6a). Thus,
system sustainability requires generation and regulation of NADPH levels. We tested whether the purge valve system can replace exogenously added NADPH in the production of isoprene. As shown
in Fig. 6b, the full purge valve system produces an 88.2±8.4% yield from 3 mM pyruvate. This yield is even higher than the 81.4±2.0% yield obtained if we add NADPH exogenously (Fig. 6b). If
we leave out any of the purge valve components (PDHNADPH, PDHNADH or NoxE), yields are markedly reduced. Thus, the purge valve system is clearly transportable to other synthetic
biochemistry systems. DISCUSSION Maintaining proper cofactor balance is an essential part of generating flux and providing a driving force through an enzymatic pathway. _In vivo_, the
enzymatic specificity for the cofactors NADH and NADPH are typically used to control the carbon flux through catabolic and anabolic pathways, respectively. Organisms typically sense the
reduction state of these cofactors and use this information to upregulate or downregulate catabolic and anabolic pathways to cope with environmental changes. _In vitro_ systems, however, do
not have the myriad peripheral pathways that facilitate this fine control. Moreover, the natural anabolic and catabolic specificities for NADH and NADPH complicate _in vitro_
biotransformations. Synthetic biochemistry systems have often dealt with these problems by careful considerations of cofactor stoichiometry in pathway design, through the use of expensive
sacrificial metabolites, re-engineering enzymes so that only a single cofactor type is needed, adding excess cofactors, or constantly adding intermediates to the reaction mix to sustain the
process. In this work, we created a robust node of control to balance the production and consumption of NADPH and NADH in a self-regulating and self-balancing manner. To our knowledge, this
is the first _in vitro_ pathway that maintains cofactor balance without requiring adherence to stoichiometry in the generation and utilization of cofactors to ensure carbon flux. In part
because the system can sustain high levels of NADPH, we can drive the transformation to near completion, converting pyruvate to either PHB or isoprene at nearly 100% of the theoretical
yield. Moreover, the high yields in our system are robust to 10-fold variations in cofactor levels. Ultimately it will be necessary to expand this pathway to incorporate the conversion of
low-cost substrates such as glucose or other sugars into pyruvate, which would involve the glycolysis pathway or parts of the glycolysis pathway. Indeed a synthetic biochemistry system
employing glycolysis has been demonstrated previously5. Building more complex compounds from acetyl-CoA such as fatty acids, polyketides and other isoprenoids will also require the
incorporation and recycling of ATP. Thus, it would be desirable to develop simple methods to regulate the ATP/ADP levels within the context of synthetic biochemistry. ATP regulation will be
an interesting challenge for future synthetic biochemistry pathway designs. Ultimately, developing regulatory systems similar to the purge valve employed here will free synthetic
biochemistry system design from having to consume the high-energy cofactors during the anabolic phase in perfect stoichiometric balance. Thus, our approach can help diversify the chemical
targets of synthetic biochemistry. METHODS MATERIALS Miller Luria-Bertani (LB) media or Miller LB-agar (BD Difco) was used for growth of bacterial strains in liquid or solid media. _E. coli_
BL21-Gold(DE3) (B, F-, _ompT, hsdS_B, (rB−,mB−), _dcm+,Tet__r_,_gal_λ, (DE3) _endA Hte_) (Agilent) was used as a host for both cloning and expression of recombinant proteins using pET
vectors. _E. coli_ TOP10(DE3) (F- mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 nupG recA1 araD139 Δ(ara-leu)7697 galE15 galK16 rpsL(StrR) endA1 λ−) was used for expression of recombinant
proteins from the pBAD/p15A vector37. Plasmids pET28a(+) and pET22b(+) were purchased from Novagen. HotStart Taq Mastermix (Denville) was used for gene amplification from genomic or plasmid
DNA. _Phusion_ DNA polymerase (Finnizymes), Taq DNA ligase (MCLab), and T5 Exonuclease (Epicenter) were purchased separately and used to make the assembly master mix (AMM) used for cloning
(ref). ATP, (±)-α-lipoic acid, pyruvate, coenzyme A and NAD+ were from Sigma. PLASMID CONSTRUCTION The expression plasmids for the PHB enzymes were constructed from the pET28a plasmid
backbone using the Nde1- and Sac1-cut sites to produce constructs with an N-terminal 6 × His tag for purification. The genes encoding acetyl-CoA acetyltraferase (phaA; YP_725941) and
acetoacetyl-CoA reductase (phaB; YP_725942)23 were amplified and cloned from _Ralstonia eutropha_ genomic DNA. The gene encoding PHB synthase38,39 (phaC; HE_610111) was synthesized and codon
optimized for expression in an _E. coli_ host at Life Technologies before being subcloned into the pET28a expression vector. For the isoprene pathway, the constructs were the same as
described in reference30. _E. coli_ BL21-Gold cells were used as the host strain for enzyme expression. All enzymes were expressed in LB media supplemented with 50 μg ml−1 kanamycin and were
induced with 0.2 mM isopropyl-β-D-1-thiogalactopyranoside added to the culture at the end of log phase growth. The phaA, phaB, MVK, PMVK and IspS were induced at 37 °C overnight and phaB,
THL/HMGR, HMGS and IDI were induced at 18 °C overnight. The phaC was induced at 25 °C for 5 h before harvesting. ENZYME PURIFICATION Cells from 0.5 l of culture were harvested by
centrifugation and resuspended in 150 mM Tris pH 7.5, 100 mM NaCl. The cells were lysed on ice with sonication and the cell debris was removed by 12,000 _g_ centrifugation at 4 °C. The
supernatant was then mixed with 5 ml nickel-nitrilotriacetic acid agarose and after 30 min, the agarose slurry was loaded onto a gravity column and washed with five-column volumes of 100 mM
Tris pH 7.5, 100 mM NaCl and 15 mM imidazole. The enzyme was then eluted with 250 mM imidazole, 100 mM Tris pH 7.5. The resulting enzyme was dialysed into 50 mM Tris pH 7.5, 50 mM NaCl and
stored at 4 °C. EXPRESSION VECTORS FOR PDH SUBUNITS AND _E. COLI_ LPLA The E1, E2 and E3 domains were all amplified separately from _G. stearothermophilus_ genomic DNA (ATCC) using primers
that contained 15–20 bp complementary to the gene and 15–20 bp complementary to the multiple cloning site in the vector where the gene would be placed. The genes encoding E1α and E1β were
amplified together from _G. stearothermophilus_ genomic DNA and cloned into pET28a(+) that had been digested with _Nco_I and _Xho_I. This created a tagless construct for E1 expression under
control of the T7 promoter where E1α translation uses the ribosomal binding site from the pET28 vector while E1β uses the endogenous ribosomal binding site from _G. stearothermophilus._ The
E2 and E3 domains were amplified separately and cloned into pET22b(+) digested with _Nde_I and _Xho_I or pET28a(+) digested with _Nco_I and _Xho_I, respectively, to create tagless E2 and E3
constructs. The _E. coli_ lipoate protein ligase, LplA, was amplified from _E. coli_ K12 genomic DNA and assembled into pBAD/p15A37 digested with _Xho_I and _Eco_RI to create 6 × His-LplA.
OVEREXPRESSION AND PURIFICATION OF PDH SUBUNITS AND LPLA All _E. coli_ strains were grown at 37 °C in LB-media supplemented with appropriate antibiotic (100 μg ml−1 ampicillin, 50 μg ml−1
kanamycin or 34 μg ml−1 chloramphenicol). For all constructs, 5 ml of overnight starter culture was used to inoculate 1 l of LB-media. Once the OD600 reached 0.6, 0.3 mM IPTG (pET vectors)
or 0.02% arabinose (pBAD/p15A) was added to induce protein expression. After 16 h, cells were harvested, resuspended (4 ml g−1 wet cells) in 50 mM Tris-Cl pH 7.5, 0.1 M NaCl (buffer A),
lysed by sonication and cell debris removed by centrifugation at 30,000 _g_ for 20 min. _E. coli_ lysate (25 ml) containing 6 × His-LplA was loaded onto a 3-ml nickel-nitrilotriacetic acid
resin (Qiagen), washed with 25 ml buffer A containing 10 mM imidazole and eluted with 5 ml buffer A containing 250 mM imidazole. Pure 6 × His-LplA was then stored at 4 °C until use. The
individual domains of _G. stearothermophilus_ PDH were partially purified from _E. coli_ lysates by heat before modification and reconstitution of the PDH complex. E1αβ-, E2- or
E3-containing lysates were incubated at 65 °C for 35 min to heat denature _E. coli_ proteins followed by centrifugation at 30,000_g_ for 20 min to pellet the precipitated proteins. Nearly
all of the PDH domains remain in the supernatant. Next, the E2 domain was lipoated in the heated extract by the addition of 1 mM (±)-α-lipoic acid, 2 mM ATP, 3 mM MgCl2 and 50 μg of purified
6 × His-LplA. The lipoation reaction was then allowed to proceed with gentle mixing overnight at 25 °C, yielding lipoated E2 (E2lip). After lipoation, E1αβ, E2lip and E3 were mixed in a
3:1:3 molar ratio and incubated for at least 1 h at 25 °C to form the active GsPDH complex. The GsPDH complex was then isolated by ultracentrifugation (Beckman) for 4 h at 95,000 _g_. The
resulting yellow pellet was resuspended in 20 mM Tris-Cl, pH 7.5 in 1/50 the starting volume and assayed for activity33,34,40. SDS–polyacrylamide gel electrophoresis analysis confirmed the
presence of all four domains and indicated that the preparation was >90% pure. The reconstituted complex was stored at 4 °C until use. ENZYME ACTIVITY AND OPTIMIZATION NoxE was assayed by
monitoring the oxidation of NAD(P)H at 340 nm. The assay was carried out in 100 mM Tris–HCl pH 7.5, 5 mM MgCl2, 5 mM KCl and 0.2 mM NAD(P)H. WT and mutant PDH were assayed by monitoring the
reduction of NAD(P)+ at 340 nm. The assay was carried out in 50 mM Tris pH 7.5, 5 mM MgCl2, 5 mM pyruvate, 1 mM CoA and 0.5 mM of NAD(P)+. PhaC was assayed by monitoring the absorbance of
the hydrolysis of the thioester bond of the substrate 3HBCoA at 235 nm. The assay was carried out in 100 mM Tris pH 7.5, 5 mM MgCl2 and 0.15 mM 3HBCoA. Activity of NADPH-consuming isoprene
pathway enzymes were monitored with the decrease of absorbance at 340 nm. Activity of ATP-consuming enzymes were monitored with a coupled assay system with pyruvate kinase and lactate
dehydrogenase at 340 nm. The amount of each enzyme in the reconstituted isoprene pathway described below is show in Supplementary Table 2. FINAL PHB REACTION CONDITIONS AND ANALYSIS The
optimized self-sustaining reaction for the biotransformation of pyruvate to PHB was composed of 250 mM Tris pH 7.5, 5 mM MgCl, 5 mM KCl, 0.5 mM CoA, 0.1 mM NAD+, 0.5 mM NADP+, 50 mM
pyruvate, 2 μg PDHNADH, 76 μg PDHNADPH, 23 μg phaA, 14 μgphaB and 32 μg phaC in a final reaction volume of 200 μl. The reactions were initiated with the addition of pyruvate, which was left
out of the initial mixture. All PHB reactions were performed at room temperature. For testing the autoregulatory behaviour of the purge valve, some enzyme concentrations were sub-optimal: 5
μg phaA, 2.5 μg phaB and 1.9 μg phaC. To assay for PHB, the reactions were lyophilized and incubated with 1 ml chloroform, 0.45 ml methanol and 0.05 ml H2SO4 to hydrolyse the polymer and
generate methyl 3-hydroxybutyrate. The reactions were extracted with 0.5 ml water and 1 μl of the chloroform layer was applied to a 0.25-micron HP-Innowax column using a HP 5890 Series II
gas chromatogram. The gas chromatography method used an injection temperature that was held at 35 °C for 5 min before it was increased to 275 °C over 40 min. The peak intensities were
compared with an authentic standard to assess concentrations. ISOPRENE REACTION CONDITIONS AND ANALYSIS _In vitro_ production of isoprene was performed as described previously30 with the
following changes. 200 μl reactions were set up in 2-ml gas-tight vials containing enzymes, 3 mM pyruvate, 15 mM ATP, 0.6 mM CoA, 0.2 mM NAD+, 0.4 mM NADP+ (or 5 mM NADPH), 10 mM MgCl2, 20
mM KCl and 0.1 mM thiamine pyrophosphate in 100 mM Tris-Cl pH 8.5 and incubated at 32 °C for 18 h. Isoprene production was monitored by direct sampling of 100 μl of the headspace using a
100-μl gas-tight syringe. The headspace was analysed by GC-FID (HP5980II) equipped with a GS-GasPro column (0.32 mm × 30 m, Agilent) as described previously30. The carrier gas was helium
with a flow rate of 5 ml min−1. The oven temperature was kept at 120 °C for 3 min then raised to 230 °C at 5 C min−1 then to 250 °C at 20 °C min−1 and held for at 250 °C for 9 min. The inlet
and detector temperatures were kept at 270 and 330 °C, respectively. The amount of isoprene produced was quantified by comparison with a standard curve of various isoprene concentrations
sampled in the same manner. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Opgenorth, P. H. _et al_. A synthetic biochemistry molecular purge valve module that maintains redox balance.
_Nat. Commun._ 5:4113 doi: 10.1038/ncomms5113 (2014). REFERENCES * Zhang, Y. H. P. Simpler is better: high-yield and potential low-cost biofuels production through cell-free synthetic
pathway biotransformation (SyPaB). _ACS Catal._ 1, 998–1009 (2011). Article CAS Google Scholar * Hold, C. & Panke, S. Towards the engineering of in vitro systems. _J. R. Soc.
Interface_ 6, (Suppl 4)S507–S521 (2009). Article CAS Google Scholar * Rollin, J. A., Tam, T. K. & Zhang, Y. H. P. New biotechnology paradigm: cell-free biosystems for
biomanufacturing. _Green Chem._ 15, 1708–1719 (2013). Article CAS Google Scholar * Algar, E. M. & Scopes, R. K. Studies on cell-free metabolism—ethanol-production by extracts of
zymomonas-mobilis. _J. Biotechnol._ 2, 275–287 (1985). Article CAS Google Scholar * Welch, P. & Scopes, R. K. Studies on cell-free metabolism—ethanol-production by a yeast glycolytic
system reconstituted from purified enzymes. _J. Biotechnol._ 2, 257–273 (1985). Article CAS Google Scholar * Bujara, M., Schumperli, M., Billerbeek, S., Heinemann, M. & Panke, S.
Exploiting cell-free systems: implementation and debugging of a system of biotransformations. _Biotechnol. Bioeng._ 106, 376–389 (2010). CAS PubMed Google Scholar * Bujara, M.,
Schumperli, M., Pellaux, R., Heinemann, M. & Panke, S. Optimization of a blueprint for in vitro glycolysis by metabolic real-time analysis. _Nat. Chem. Biol._ 7, 271–277 (2011). Article
CAS Google Scholar * Ye, X. et al. Synthetic metabolic engineering-a novel, simple technology for designing a chimeric metabolic pathway. _Microb. Cell. Fact._ 11, 120 (2012). Article
CAS Google Scholar * Ye, X., Honda, K., Morimoto, Y., Okano, K. & Ohtake, H. Direct conversion of glucose to malate by synthetic metabolic engineering. _J. Biotechnol._ 164, 34–40
(2013). Article CAS Google Scholar * Yu, X. Y., Liu, T. G., Zhu, F. Y. & Khosla, C. In vitro reconstitution and steady-state analysis of the fatty acid synthase from Escherichia coli.
_Proc. Natl Acad. Sci. USA_ 108, 18643–18648 (2011). Article CAS ADS Google Scholar * Jewett, M. C. & Swartz, J. R. Rapid expression and purification of 100 nmol quantities of
active protein using cell-free protein synthesis. _Biotechnol. Prog._ 20, 102–109 (2004). Article CAS Google Scholar * Jewett, M. C., Calhoun, K. A., Voloshin, A., Wuu, J. J. &
Swartz, J. R. An integrated cell-free metabolic platform for protein production and synthetic biology. _Mol. Syst. Biol._ 4, 220 (2008). Article Google Scholar * Schultheisz, H. L.,
Szymczyna, B. R., Scott, L. G. & Williamson, J. R. Pathway engineered enzymatic de novo purine nucleotide synthesis. _ACS Chem. Biol._ 3, 499–511 (2008). Article CAS Google Scholar *
Schultheisz, H. L., Szymczyna, B. R., Scott, L. G. & Williamson, J. R. Enzymatic _de novo_ pyrimidine nucleotide synthesis. _J. Am. Chem. Soc._ 133, 297–304 (2011). Article CAS Google
Scholar * Zhang, Y. H. P., Evans, B. R., Mielenz, J. R., Hopkins, R. C. & Adams, M. W. W. High-yield hydrogen production from starch and water by a synthetic enzymatic pathway. _PLoS
ONE_ 2, e456 (2007). Article ADS Google Scholar * Ye, X. H. et al. Spontaneous high-yield production of hydrogen from cellulosic materials and water catalyzed by enzyme cocktails.
_ChemSusChem_ 2, 149–152 (2009). Article CAS Google Scholar * Woodward, J., Orr, M., Cordray, K. & Greenbaum, E. Biotechnology—enzymatic production of biohydrogen. _Nature_ 405,
1014–1015 (2000). Article CAS ADS Google Scholar * Krutsakorn, B. et al. In vitro production of n-butanol from glucose. _Metab. Eng._ 20, 84–91 (2013). Article CAS Google Scholar *
Guterl, J. K. et al. Cell-free metabolic engineering: production of chemicals by minimized reaction cascades. _ChemSusChem_ 5, 2165–2172 (2012). Article CAS Google Scholar * Chen, G.-Q.
in_Plastics from Bacteria: Natural Functions and Applications_ Vol. 14 _Microbiology Monographs_ ed Chen G. Q. 121–132Springer (2010). * Kawada, J., Lutke-Eversloh, T., Steinbuchel, A. &
Marchessault, R. H. Physical properties of microbial polythioesters: characterization of poly(3-mercaptoalkanoates) synthesized by engineered _Escherichia coli_. _Biomacromolecules_ 4,
1698–1702 (2003). Article CAS Google Scholar * Dawes, E. A. Polyhydroxybutyrate—an intriguing bio-polymer. _Biosci. Rep._ 8, 537–547 (1988). Article CAS Google Scholar * Satoh, Y.,
Tajima, K., Tannai, H. & Munekata, M. Enzyme-catalyzed poly(3-hydroxybutyrate) synthesis from acetate with CoA recycling and NADPH regeneration in vitro. _J. Biosci. Bioeng._ 95, 335–341
(2003). Article CAS Google Scholar * Yang, J. et al. Enhancing production of bio-isoprene using hybrid MVA pathway and isoprene synthase in _E. coli_. _PLoS ONE_ 7, e33509 (2012).
Article CAS ADS Google Scholar * Kesselmeier, J. & Staudt, M. Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology. _J. Atmos. Chem._ 33, 23–88
(1999). Article CAS Google Scholar * Lindberg, P., Park, S. & Melis, A. Engineering a platform for photosynthetic isoprene production in cyanobacteria, using Synechocystis as the
model organism. _Metab. Eng._ 12, 70–79 (2010). Article CAS Google Scholar * Peralta-Yahya, P. P., Zhang, F. Z., del Cardayre, S. B. & Keasling, J. D. Microbial engineering for the
production of advanced biofuels. _Nature_ 488, 320–328 (2012). Article CAS ADS Google Scholar * Yang, J. M. et al. Bio-isoprene production using exogenous MVA pathway and isoprene
synthase in _Escherichia coli_. _Bioresour. Technol._ 104, 642–647 (2012). Article CAS Google Scholar * Xue, J. & Ahring, B. K. Enhancing isoprene production by genetic modification
of the 1-deoxy-D-xylulose-5-phosphate pathway in _Bacillus subtilis_. _Appl. Environ. Microbiol._ 77, 2399–2405 (2011). Article CAS Google Scholar * Korman, T. P. et al. A synthetic
biochemistry system for the in vitro production of isoprene from glycolysis intermediates. _Protein Sci._ 23, 576–585 (2014). Article CAS Google Scholar * Kawasaki, S., Ishikura, J.,
Chiba, D., Nishino, T. & Niimura, Y. Purification and characterization of an H2O-forming NADH oxidase from _Clostridium aminovalericum_: existence of an oxygen-detoxifying enzyme in an
obligate anaerobic bacteria. _Arch. Microbiol._ 181, 324–330 (2004). Article CAS Google Scholar * Geueke, B., Riebel, B. & Hummel, W. NADH oxidase from _Lactobacillus brevis_: a new
catalyst for the regeneration of NAD. _Enzyme Microb. Technol._ 32, 205–211 (2003). Article CAS Google Scholar * Bocanegra, J. A., Scrutton, N. S. & Perham, R. N. Creation of an
nadp-dependent pyruvate-dehydrogenase multienzyme complex by protein engineering. _Biochemistry_ 32, 2737–2740 (1993). Article CAS Google Scholar * Richter, N., Zienert, A. & Hummel,
W. A single-point mutation enables lactate dehydrogenase from _Bacillus subtilis_ to utilize NAD(+) and NADP(+) as cofactor. _Eng. Life Sci._ 11, 26–36 (2011). Article CAS Google Scholar
* Gao, H., Tiwari, M. K., Kang, Y. C. & Lee, J. K. Characterization of H2O-forming NADH oxidase from Streptococcus pyogenes and its application in L-rare sugar production. _Bioorg. Med.
Chem. Lett._ 22, 1931–1935 (2012). Article CAS Google Scholar * Gerngross, T. & Martin, D. Enzyme-catalyzed synthesis of poly [(R)-(-)-3-hydroxybutyrate]: formation of macroscopic
granules in vitro. _Proc. Natl Acad. Sci. USA_ 92, 6279–6283 (1995). Article CAS ADS Google Scholar * Massey-Gendel, E. et al. Genetic selection system for improving recombinant membrane
protein expression in E-coli. _Protein Sci._ 18, 372–383 (2009). Article CAS Google Scholar * Rehm, B. H. A. Polyester synthases: natural catalysts for plastics. _Biochem. J._ 376, 15–33
(2003). Article CAS Google Scholar * Sheu, D. S., Chen, W. M., Lai, Y. W. & Chang, R. C. Mutations derived from the thermophilic polyhydroxyalkanoate synthase PhaC enhance the thermo
stability and activity of PhaC from Cupriavidus necator H16. _J. Bacteriol._ 194, 2620–2629 (2012). Article CAS Google Scholar * Kim, D. J. et al. Crystal structure of lipoate-protein
ligase A bound with the activated intermediate—Insights into interaction with lipoyl domains. _J. Biol. Chem._ 280, 38081–38089 (2005). Article CAS Google Scholar Download references
ACKNOWLEDGEMENTS We thank members of the Bowie lab for critical reading of this manuscript. The work was supported by the DOE grant DE-FC02-02ER63421 to J.U.B. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Department of Chemistry and Biochemistry, UCLA-DOE Institute for Genomics and Proteomics, Molecular Biology Institute, University of California, Los Angeles, 90095-1570,
California, USA Paul H. Opgenorth, Tyler P. Korman & James U. Bowie * Boyer Hall, UCLA, 611 Charles E Young Drive East, Los Angeles, 90095-1570, California, USA James U. Bowie Authors *
Paul H. Opgenorth View author publications You can also search for this author inPubMed Google Scholar * Tyler P. Korman View author publications You can also search for this author inPubMed
Google Scholar * James U. Bowie View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS P.H.O., T.P.K. and J.U.B. designed the experiments,
analysed experimental results and wrote the maunscript. P.H.O. and T.P.K. conducted the experiments. CORRESPONDING AUTHOR Correspondence to James U. Bowie. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figures 1-2, Supplementary Tables 1-4 and Supplementary
References (PDF 423 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Opgenorth, P., Korman, T. & Bowie, J. A synthetic biochemistry molecular
purge valve module that maintains redox balance. _Nat Commun_ 5, 4113 (2014). https://doi.org/10.1038/ncomms5113 Download citation * Received: 19 December 2013 * Accepted: 14 May 2014 *
Published: 17 June 2014 * DOI: https://doi.org/10.1038/ncomms5113 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative








