- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Plants control CO2 uptake and water loss by modulating the aperture of stomata located in the epidermis. Stomatal opening is initiated by the activation of H+-ATPases in the
guard-cell plasma membrane. In contrast to regulation of H+-ATPase activity, little is known about the translocation of the guard cell H+-ATPase to the plasma membrane. Here we describe the
isolation of an _Arabidopsis_ gene, _PATROL1_, that controls the translocation of a major H+-ATPase, AHA1, to the plasma membrane. _PATROL1_ encodes a protein with a MUN domain, known to
mediate synaptic priming in neuronal exocytosis in animals. Environmental stimuli change the localization of plasma membrane-associated PATROL1 to an intracellular compartment. Plasma
membrane localization of AHA1 and stomatal opening require the association of PATROL1 with AHA1. Increased stomatal opening responses in plants overexpressing _PATROL1_ enhance the CO2
assimilation rate, promoting plant growth. SIMILAR CONTENT BEING VIEWED BY OTHERS _ARABIDOPSIS_ COP1 GUIDES STOMATAL RESPONSE IN GUARD CELLS THROUGH PH REGULATION Article Open access 05
February 2024 DICHOTOMY OF THE BSL PHOSPHATASE SIGNALING SPATIALLY REGULATES MAPK COMPONENTS IN STOMATAL FATE DETERMINATION Article Open access 04 May 2022 THE ATMYB60 TRANSCRIPTION FACTOR
REGULATES STOMATAL OPENING BY MODULATING OXYLIPIN SYNTHESIS IN GUARD CELLS Article Open access 11 January 2022 INTRODUCTION Stomatal pores, formed by pairs of guard cells, serve as major
gateways for gas exchange between plants and their environments1. Opening of stomata is stimulated by low CO2 concentrations and light, whereas stomatal closure occurs in response to high
CO2, darkness and shortage of water. Guard cells integrate these signals and appropriately adjust the stomatal pore apertures to optimize growth performance2,3. Stomatal opening is driven by
an increase in guard-cell turgor when plasma membrane H+-ATPases are activated through phosphorylation and induce membrane hyperpolarization facilitating K+ entry into the guard cells4,5.
Although the activity of plasma membrane H+-ATPases is modulated by various physiological signals, there is little evidence that these factors alter the abundance of H+-ATPase in the plasma
membrane6. Here we describe a Munc13 ortholog named PATROL1 that in _Arabidopsis_ controls the tethering of an H+-ATPase, AHA17, to the plasma membrane, and demonstrate that PATROL1 function
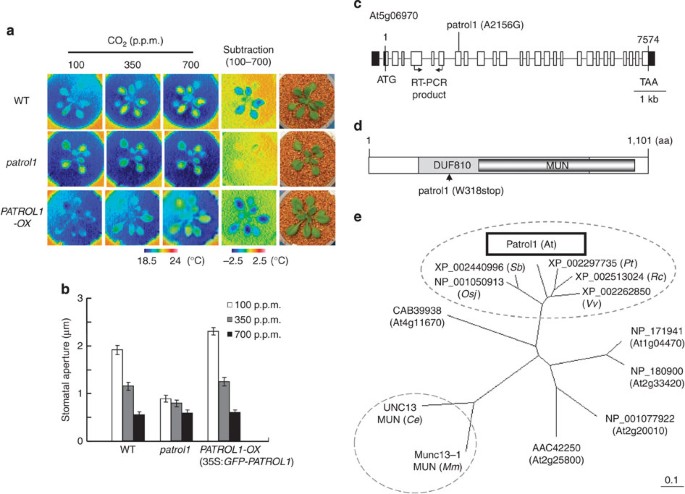
is essential for stomatal opening in response to low [CO2] and light. RESULTS THE _PATROL1_ MUTATION IMPAIRS THE STOMATAL OPENING RESPONSE Leaf temperature acts as a convenient indicator of
transpiration and can help detect mutants with altered stomatal control8,9. _Arabidopsis ht_ mutants were identified by higher leaf temperature than the wild type (WT) under low CO2
conditions, as visualized by infrared imaging9 (Fig. 1a). One of them, a recessive mutant originally referred to as _ht2_ and here renamed _patrol1_ (_proton ATPase translocation control
1)_, was impaired in CO2-dependent leaf temperature change (Fig. 1a) as well as in stomatal opening in response to low [CO2] (Fig. 1b). The stomatal density of 3-week-old leaves was similar
in WT and _patrol1_ plants (136±23.4 and 132±23.6 stomata per mm2 in WT and _patrol1_, respectively; means±s.d.; _n_=20 leaves). Thus, the high leaf temperature at low [CO2] in _patrol1_
could not be explained by stomatal density, but by a reduction of stomatal aperture. _PATROL1_ ENCODES A PROTEIN WITH MUN DOMAIN The _PATROL1_ locus was mapped to a location between the
Cereon single-nucleotide polymorphism marker, CER438918, and the original molecular marker moj9-47976, on chromosome 5 (Supplementary Fig. S1). Sequencing of this 47-kb region revealed that
At5g06970 harboured a point mutation at nucleotide 2,156 that resulted in the exchange of Trp-318 for a stop codon (Fig. 1c,d). The 3.8-kb _PATROL1_ cDNA fragment expressed under the control
of the CaMV 35S promoter complemented the smaller mutant phenotype and restored the CO2 response in stably transformed _patrol1_ (35S:_PATROL1_) (Supplementary Fig. S2). This indicates that
_PATROL1_ is At5g06970, which encodes an uncharacterized protein with a Domain of Unknown Function 810 (DUF810) (Fig. 1c; InterPro; http://www.ebi.ac.uk/interpro/) of 1,101 amino acids.
Plant proteins that have a MUN domain have been classified as DUF810 (Fig. 1d). Munc13 proteins are crucial components in neurotransmitter release in animals, controlling the priming of
synaptic vesicles to the release-ready state10,11. Intracellular membrane fusion is generally dependent on the activities of SNARE complexes12. A large α-helical domain of mammalian
Munc13-1, the MUN domain, dramatically accelerates the transition from the closed Syntaxin-1/Munc18-1 complex to the SNARE complex13, and is sufficient for the activity of Munc13-1 in
synaptic vesicle priming14. MUN domains are found in diverse proteins from animals, plants and fungi15,16. Munc13 proteins in animals have extensive conserved sequences and domains in
addition to the MUN domain16. We compared the amino-acid sequences of the MUN domains of two representative Munc13 proteins. The animal MUN domains exhibit weak amino-acid identities with
PATROL1 (Fig. 1e; 16% in Unc13-MUN (_C. elegans_) and 8% in Munc13-MUN (_Mus musculus_)). The sequence identities of _PATROL1_ with five other _Arabidopsis_ DUF810 members are not high
(17–32%; Fig. 1e, Supplementary Fig. S3). On the other hand, higher plants including monocots and dicots have PATROL1 orthologues with highly conserved homologies distributed across the
entire sequences (identities; 66–76%; Fig. 1e, Supplementary Fig. S3), suggesting an essential function. Computational biology and available three-dimensional (3D) structures indicate
structural similarity between MUN domains of Munc13-related proteins including PATROL1 and other distantly related vesicle tethering factors such as the Exocyst, GARP, COG and Dsl1p
complexes16,17. This suggests that PATROL1 may have a conserved function in intracellular membrane fusion. EXPRESSION PATTERN OF _PATROL1_ IN _ARABIDOPSIS_ To examine the expression patterns
of _PATROL1_, we used the PATROL1 promoter to drive expression of the GUS reporter (p_PATROL1_::_GUS_; Supplementary Fig. S4a–f), and RT-PCR analysis with gene-specific primers (Fig. 1c,
Supplementary Fig. S4g,h). _PATROL1_ was expressed in the whole plant, including guard cells (Supplementary Fig. S4). Global gene expression analysis of microarrays indicated that other
DUF810 member genes in _Arabidopsis_ were expressed mainly in floral organs (_At1g04470_ and _At2g33420_) or in the whole plant (_At2g20010_, _At2g25800_ and _At4g11670_), similar to
_PATROL1_18. Two closely related genes (_At2g33420_ and _At4g11670_) were expressed especially in mature pollen and pollen tubes, suggesting roles in pollen germination and tube growth19.
Compared to other DUF810 member genes with pleiotropic expression, PATROL1 expression levels appeared relatively high18, and the loss-of-function mutation in _PATROL1_ impaired stomatal
movement (Fig. 1b) and caused growth retardation (Supplementary Fig. S2), raising the possibility that _PATROL1_ has a major function in stomatal response and plant growth. ENVIRONMENTAL
CONDITIONS ALTER PATROL1 SUBCELLULAR DISTRIBUTION We generated transgenic plants expressing green fluorescent protein (GFP) fused to the N terminus of the full-length PATROL1 protein
(GFP–PATROL1) to determine the intracellular localization of PATROL1. The GFP–PATROL1 rescued the _patrol1_ mutant phenotype; the plants showed increased sensitivity to low [CO2] due to
overexpression of the transgenes (Fig. 1a,b, Supplementary Fig. S2). The lipophilic dye FM4-64 is internalized in living cells through endocytosis and can be used to visualize the endocytic
pathway from the plasma membrane to the tonoplast20,21. Two minutes after the transfer of GFP–PATROL1 transgenic plants into FM4-64 buffer, the fluorescent dye exhibited a punctate pattern
typical of endosomes with little or no vacuolar staining in hypocotyl epidermal cells (Fig. 2a,b). The FM4-64 signal partially colocalized with the GFP–PATROL1 fluorescence signal (Fig. 2b),
indicating that GFP–PATROL1 resides in an endosomal compartment. This finding paralleled results from mouse brain cells, where signals from Munc13-1-EYFP and FM4-64 also colocalized22. When
incubated in the dark for 72 h, numerous small structures showing GFP–PATROL1 fluorescence were observed in the guard cells of leaves in transgenic plants (Fig. 2c,d; dark); their number
decreased after 30 min in the light (Fig. 2c,d; light for 30 min). The structures were rare in the guard cells of well-watered and irradiated plants, when most of the GFP–PATROL1
fluorescence was detected in close proximity to the plasma membrane (Fig. 2e,f; detached for 0 min). Within 30 min after detachment of the leaves, small fluorescent structures were observed
inside the cell (Fig. 2e,f; detached for 30 min). These findings suggest that GFP–PATROL1 moved reversibly between the cytoplasm and the plasma membrane depending on environmental
conditions. MUTATION OF _PATROL1_ CAUSES ALTERED LOCALIZATION OF AHA1 If PATROL1 has a role in intracellular membrane traffic, it might control the localization of ion transporters that are
crucial to stomatal opening (for example, plasma membrane H+-ATPases). Stomatal opening is initiated by hyperpolarization of the guard-cell plasma membrane caused by H+-ATPase-dependent
proton efflux5. Membrane hyperpolarization activates inward-rectifying potassium channels and induces solute influx followed by water uptake into guard cells4. _Arabidopsis_ H+-ATPase 1
(AHA1) is highly expressed in guard cells and its activation induces stomatal opening7,23. We generated transgenic plants containing a _GFP–AHA1_ fusion gene in the WT (AHA1/WT) and
_patrol1_ (AHA1/_patrol1_) background. In WT guard cells, GFP–AHA1 was localized mainly at the plasma membrane (Fig. 3a) as expected24. The internalization rate calculated from the number of
guard cells that exhibited small fluorescent structures in their cytosol was 80% in AHA1/_patrol1_ transgenic plants (Fig. 3a,b). This rate was significantly higher than that of AHA1/WT
transgenic plants (8%; Fig. 3a,b), indicating that PATROL1 affects AHA1 localization to the plasma membrane. The slow anion channel SLAC1 contributes to anion efflux from guard cells and
causes membrane depolarization, resulting in stomatal closure25,26. We compared the distribution of SLAC1–GFP fluorescence in transgenic WT and _patrol1_ plants. Little internalization was
observed in the guard cells of SLAC1–GFP transgenic plants even in the _patrol1_ background (SLAC1/WT, 1%; SLAC1/_patrol1_, 3%; Fig. 3a,b). We examined another transporter, aquaporin PIP1a,
which is localized in the plasma membrane of guard cells27. GFP–PIP2a has been used as a plasma membrane protein marker28, and we also detected GFP fluorescence in the plasma membrane
(GFP–PIP2a/WT, Fig. 3a,b). In the _patrol1_ background, the internalization rate of GFP–PIP2a was slightly increased but remained low (PIP2a/WT, 0%; PIP2a/_patrol1_, 8.6%; Fig. 3a,b). These
results indicated that PATROL1 is target-selective, as it is required for the direction of AHA1 to the plasma membrane, but less so for SLAC1 or PIP2a. We also examined the localization of
the inward-rectifying potassium channel, KAT1, in _patrol1_ mutants. This voltage-gated channel is activated by hyperpolarization, and mediates the influx of potassium into guard cells29. We
could only slightly detect punctate structures of KAT1–GFP fluorescence in _patrol1_ mutants, indicating that PATROL1 does not affect KAT1 localization to the plasma membrane (KAT1/WT,
0.6%; KAT1/_patrol1_, 1.1%; Supplementary Fig. S5). To our knowledge, the KAT1–GFPfluorescence in WT chloroplast envelopes that we detected has never been discussed before, but some figures
in a previous report30 showed similar localization. Among the four GFP-tagged plasma membrane-localized proteins in our transgenic plants, the fluorescence intensity of GFP–AHA1 was the
strongest. As overexpressed membrane-targeted GFP fusion proteins have a propensity to form organelle aggregates as a consequence of oligomerization31, we examined whether the increased
internalization rate of the GFP–AHA1 in the _patrol1_ mutant was caused by GFP. The monomeric red fluorescent protein (mRFP) contains 33 mutations compared to DsRed and was designed as a
non-oligomerizing, true monomer32. The RFP-tagged AHA1 in the _patrol1_ mutant appeared as numerous punctate structures in the cytosol (Fig. 3c), while it exhibited an extremely low
fluorescence intensity in the plasma membrane (Fig. 3d,e), indicating apparent mislocalization of AHA1. We further attempted to identify plasma membrane H+-ATPases in the epidermis of
rosette leaves using anti-H+-ATPase antibodies (anti-AHA) (Supplementary Fig. S6). The mean value of the fluorescent intensity from immunohistochemical staining in _patrol1_ was slightly but
not statistically significantly lower than in the WT (266.1 in arbitrary unit in WT, 288.3 in arbitrary unit in _patrol1_, respectively; _P_=0.24, Supplementary Fig. S6). One reason why no
clear difference could be observed is that this method has the potential to detect not only plasma membrane AHA but also AHA in endosomes. Actually, intracellular localization of the
H+-ATPase has been found by this method, probably due to an artifact caused by the fixation for immunohistochemical detection33. It also seemed extremely difficult to distinguish endosomal
AHA from plasma membrane AHA in guard cell protoplast proteins and to quantify their amounts. In order to estimate the amount of functional H+-ATPases in the plasma membrane of guard cells,
we investigated stomatal apertures when H+-ATPases were compulsorily activated. The fungal phytotoxin fusicoccin (FC) induces irreversible stomatal opening by the continuous activation of
the plasma membrane H+-ATPases due to inhibition of their dephosphorylation in guard cells5,34. Though _Arabidopsis_ has at least 11 plasma membrane H+-ATPases and all of which are expressed
in guard cells23, the stomatal response to FC was severely impaired in _patrol1_ (Fig. 3f;<0.5, unpaired two-tailed _t_-test), suggesting that the recruitment of functional H+-ATPases to
the plasma membrane was disturbed by the mutation. Thus, PATROL1 may have a crucial role in the recruitments of not only AHA1 but also of the other H+-ATPases. 35S:_PATROL1_ and
35S:_GFP–PATROL1_ transgenic plants showed rather strong FC responses indicating increased AHA level in the plasma membrane (Fig. 3f). To obtain further insights into the relationship
between PATROL1 and AHA1, we generated transgenic plants co-expressing GFP–PATROL1 and RFP–AHA1. In hypotonic conditions, which lead to stomatal opening, RFP–AHA1 was located in the plasma
membrane, while GFP–PATROL1 seemed to line the membrane (Fig. 4a). In hypertonic conditions, which induce stomatal closure, RFP–AHA1 partially remained in the plasma membrane and GFP–PATROL1
seemed to support it. They resided on the plasma membrane–cell wall connection spots, that is, the tips of Hechtian strands in a plasmolyzed guard cells (Fig. 4b, arrows). These
relationships suggest that PATROL1 contributes to H+-ATPase localization to the plasma membrane. STOMATAL OPENING AND CO2 UPTAKE IN _PATROL1-OX_ PLANTS To characterize the function of
_PATROL1_, we compared the phenotypes of the _patrol1_ mutant and the _PATROL1_-overexpressing line (Supplementary Fig. S2c). 35S:_GFP–PATROL1_ (hereafter _PATROL1-OX_) exhibited functional
complementation of _patrol1,_ including restored sensitivity to FC (Fig. 3f) and low [CO2] (Fig. 1a, Supplementary Fig. S2b)_. PATROL1-OX_ exhibited lower leaf temperature under low [CO2]
than the WT due to enhanced stomatal opening (Fig. 1a,b). The rises in stomatal conductance in response to low [CO2] and light were more rapid and reached higher maximum values (sometimes
followed by oscillations) in _PATROL1-OX_ than in the WT, whereas those in _patrol1_ were slow and ceased at low values (Fig. 5a,c). Enhanced stomatal opening responses in _PATROL1-OX_
plants may contribute to higher CO2 assimilation rates even under low [CO2] (Fig. 5a–d). Under normal or increased [CO2], _PATROL1-OX_ plants exhibited similar but slightly higher stomatal
conductance compared to the WT. However, this small difference may be sufficient to elevate the CO2 assimilation rate (Fig. 5a,b). We examined the response to desiccation stress in
_PATROL1-OX_ plants. The rate of transpirational water loss, evaluated as the fresh weight decrease of the excised _PATROL1-OX_ leaves, resembled that of the WT. This was unlike the response
found in the ABA-deficient mutant _aba2-1_35 that showed excessive water loss owing to constitutive stomatal opening (Fig. 5e). These results suggested that _PATROL1-OX_ plants control the
stomatal aperture efficiently; they could open their stomata wider than the WT to maintain higher CO2 assimilation rates, and close them to prevent extra water loss depending on the
environmental conditions (Fig. 5). _PATROL1-OX_ PLANTS EXHIBIT ENHANCED GROWTH When grown under continuous light for 20 days, _patrol1_ plants were significantly smaller than the WT (Fig.
6a,b, analysis of variance (ANOVA), _P_=1.7 × 10−11, _post hoc_ Tukey tests, _P_=2.4 × 10−7), whereas _PATROL1-OX_ plants were bigger (in terms of above-ground fresh weight; Fig. 6a,b, _post
hoc_ Tukey tests, _P_=0.0496). Thus, plant size correlated with CO2 assimilation rate under standard [CO2] (350 p.p.m.; Fig. 5b,d). Likewise, when grown for 7 weeks under short-day
conditions (8 h light, 16 h dark), _PATROL1-OX_ plants were 32% bigger than the WT (Fig. 6d,e, ANOVA, _P_=1.17 × 10−4, _post hoc_ Tukey tests, _P_=1.2 × 10−3), although _patrol1_ rosettes
reached the size of WT ones (Fig. 6d,e, _post hoc_ Tukey tests, _P_=0.75). Compared to the WT, shorter and longer inflorescence stems were observed in _patrol1_ and _PATROL1-OX_,
respectively, when grown under continuous light for a month (Fig. 6c). Similar results were obtained when plants were grown first under short-day conditions for 7 weeks and then shifted to
long-days to induce flowering for 2 more weeks (Fig. 6f). Taken together, PATROL1 affects not only stomatal movement but also leaf growth and stem elongation. DISCUSSION In animals, Munc13
proteins are important for synaptic exocytosis and for cytotoxic granule exocytosis in NK cells36. Animal Munc13 proteins possess several conserved domains including Ca2+-binding sites
and/or domains required for homodimerization36. Metazoan and fungal MUN domain-containing proteins carry C2 domains suggesting roles in regulating Ca2+-dependent exocytosis. In plants, MUN
domain-containing proteins are relatively smaller and have no known domains other than MUN16. This might suggest that Munc13-like proteins in plants do not need Ca2+ binding or need other
Ca2+ binding components for its activation. In plants, the functions of Munc13-like genes are generally unknown. In this study, we showed that one of the _Arabidopsis_ Munc13-like genes,
_PATROL1_, has a role in stomatal responses and growth by regulation of the plasma membrane H+-ATPase. We found that PATROL1 orthologues in higher plants have high identity and similarity to
AtPATROL1 including a part adjacent to the MUN domain (Supplementary Fig. S4). These parts may be required for functions unique to plants that need to integrate signals received from the
environment including light, CO2 and humidity. H+-ATPases are known to contribute to stomatal opening, cell elongation, and the growth of root hairs and pollen tubes5. Some of the DUF810
family genes have been reported to be expressed in pollen18, suggesting functions in pollen germination and tube growth19. Thus, these genes may also have a role in H+-ATPase tethering on
the plasma membrane in pollen tubes. Endosomes traffic biosynthetic cargo and recycle endocytosed plasma membrane components back to the plasma membrane. We showed that PATROL1 resides in
the endosome and moves to and from the plasma membrane in response to environmental stimuli (Fig. 2). Under light and well-watered conditions that induce stomatal opening, PATROL1 mainly was
found close to the plasma membrane, whereas under dark or dry conditions that induce stomatal closure, PATROL1 moved away from the plasma membrane (Fig. 2). The AHA1 in the plasma membrane
was always associated with PATROL1 on the inside of the plasma membrane (Fig. 4). These facts are consistent with the function of PATROL1 in tethering H+-ATPases to the plasma membrane.
Stomatal movements by guard-cell swelling or shrinking require changes of guard-cell surface area through exocytotic addition or endocytotic retrieval of membrane37,38. When stomata open,
intracellular vesicles incorporate with the plasma membrane and PATROL1 may be carried with them to tether H+-ATPases into the plasma membrane. In plasma membrane H+-ATPases, changes in
transcriptional levels are often uncoupled from changes in protein activities and/or proton fluxes6. It is therefore likely that post-translational modifications determine H+-ATPase
activity. Phosphorylation of H+-ATPases and the phosphorylation-dependent binding of 14-3-3 protein are well-studied examples of post-translational regulation5. Modifications of the
H+-ATPase concentration in the plasma membrane are an alternative means to control total activity, but few studies addressed this alternative regulatory pathway6. A nonsense mutation just
before the MUN domain of PATROL1 (Fig. 1d) resulted in irregular pattern of AHA1 distribution (Fig. 3a–e). In addition, _patrol1_ showed defects in FC-, low CO2- and light-induced stomatal
opening (Figs 3f, 5a,c), which supports the notion that the translocation of H+-ATPases to the plasma membrane is an essential process in stomatal function that is controlled by PATROL1.
Furthermore, we showed that higher CO2 assimilation rates increase biomass production in _PATROL1_-overexpressing plants. An _Arabidopsis_ SNARE protein, syntaxin SYP121, functions in the
anchoring of K+ channels to the plasma membrane through direct physical interaction39. The _syp121_ mutant exhibits delayed stomatal opening and develops similar or smaller rosettes than the
WT depending on environmental conditions30, similar to _patrol1_. This seems to indicate that membrane traffic generally controls stomatal movement, and consequently, plant growth.
Dominant-negative Sp2 fragments of SYP121 from _Arabidopsis_ and maize do not affect the plasma membrane delivery of the H+-ATPase PMA240. Taken together, SNARE proteins select their target
plasma membrane proteins directly. PATROL1 may select its target H+-ATPases indirectly by interacting with specific SNARE proteins and control the total amount of the plasma membrane
H+-ATPases. In this study, the _patrol1_ mutation severely impaired the localization of AHA1 but did not affect that of KAT1, indicating that the target SNARE protein PATROL1 associates with
may be different from SYP121. Auxin, a plant growth hormone, inhibits endocytosis of plasma membrane proteins and increases the density of H+-ATPases in the PM41. H+-ATPase acidifies the
apoplast and loosens the cell wall leading to cell expansion42. As _PATROL1_ is expressed in the entire plant (Supplementary Fig. S4), an increased accumulation of H+-ATPases in the plasma
membrane of various cell types may contribute to the enhanced growth in _PATROL1-OX_ (Fig. 6). The constitutive activation of H+-ATPases induces stomatal opening, but plants with
continuously opened stomata often show comparatively small size and increased susceptibility to drought stress7,43. _PATROL1-OX_ exhibited normal resistance against water loss (Fig. 5e). The
ability to prevent water loss may contribute to the increased biomass production in _PATROL1-OX_. Overexpression of H+-ATPase genes does not always enhance growth because of the
downregulation of H+-ATPase activity induced by the overexpression of its gene43,44. The nature of this feedback mechanism has not been elucidated yet. Similarly, overexpression of _AHA1_
did not induce biomass increase and rather decreased biomass production in this study (Supplementary Fig. S7). On the contrary, overexpression of _PATROL1_ increased biomass even in an
_AHA1_ overexpression background (Supplementary Fig. S7). This suggests that PATROL1 is required for efficient biomass production by localizing the proper amount of H+-ATPase to the plasma
membrane without downregulating its activity. Stomatal opening in response to environmental signals in _PATROL1-_overexpressing plants was rapid (Fig. 5, Supplementary Fig. S8), suggesting
that PATROL1 may facilitate H+-ATPase activation. Highly conserved PATROL1 orthologues appear commonly in higher plants (Fig. 1e, Supplementary Fig. S3). Taken together, our findings suggest
that increases in biomass production in various plant species of interest may be achievable through manipulations of the function of _PATROL1_ and related genes. METHODS PLANT MATERIAL AND
GROWTH CONDITIONS All _Arabidopsis_ lines used in this study, including a transgenic line expressing SLAC1–GFP25, were derived from the Columbia (Col-0) background. EMS-mutagenized Col M2
seeds were purchased from Lehle Seeds (Round Rock, TX, USA). Plants were grown on solid 1/2 MS medium for 18 days in a growth chamber (constant white light of 80 μmol m–2s–1 at 22 °C, 60%
RH), and then transplanted into vermiculite pots supplemented with mineral nutrients. Under short-day condition, plants were grown on commercial soil (Supermix A, Sakata, Japan) in a growth
chamber (8 h light of 127 μmol m–2 s–1 and 16 h dark at 23 °C, 60% RH). THERMAL IMAGING Three-week-old plants were transferred to a growth cabinet (constant white light of 40 μmol m–2 s–1 at
22 °C, 43% RH) equipped with an automatic CO2 control unit (FR-SP, Koito)9. Thermal images of plants were captured under different [CO2] conditions using a thermography apparatus (TVS-8500,
Nippon Avionics)9. STOMATAL APERTURE RESPONSE ANALYSIS Three-week-old plants were incubated at the indicated [CO2] in a growth cabinet. Abaxial epidermal peels of the plants were taken from
the sixth or seventh leaf and were used immediately for aperture determination9. In FC treatment, epidermal peels were floated on a test medium containing 10 mM KCl, 10 mM Mes-KOH and 50 μM
CaCl2 (pH 6.15) and were incubated in the growth chamber. FC was added to the solution after 1 h of illumination and stomatal apertures were measured 2 h later9. The stomatal aperture was
observed in epidermal peels with a digital camera attached to a microscope (BH2, Olympus, Tokyo Japan). STOMATAL CONDUCTANCE MEASUREMENT Gas exchange was measured on the aerial part of the
24-day-old seedlings using gas exchange systems (GFS3000, Heinz Walz, Effeltrich, Germany) equipped with a 3010-A _Arabidopsis_ chamber45. The GFS3000 system was connected with a PC with
data acquisition software (GFS-Win). The _Arabidopsis_ cuvette was exposed to a light intensity of 200 μmol m–2 s–1 provided by a special artificial light (LED-Array/PAMFluorometer 3055-Fl,
Heinz Walz, Effeltrich, Germany), with relative humidity and air temperature set to 50% and 22 °C, respectively. Measurements were made every minute. TRANSGENE CONSTRUCTION For functional
complementation of the _patrol1_ mutant, a fragment containing full-length _At5g06970_ cDNA (accession number AY090450) was prepared from the RAFL 09-69-H21 clone, which was obtaind from
RIKEN BioResource Center, Tsukuba, Japan, by digestion with _Not_I and _Bam_HI and subcloning into the vector pBluescript II KS+. A _Sal_I-_Bam_HI fragment including the cDNA was cloned into
the T-DNA vector pPZP2Ha3(−)46. For the production of 35S:_GFP–PATROL1_, the 35S promoter:_GFP_ fragment with a glycine linker obtained by PCR using primers
5′-GGGCCCCTCGAGCCCCTCAGAAGACCAGAGGGC-3′ and 5′-TACCGGTAGCACCTCCACCTCCCTTAT-3′ (the glycine linker site is underlined) with pKS(+)_GFP_–_OsGK1_47 as a template, was inserted into pGEM-T Easy
vector (Promega) to produce pG-35S:_GFP_. A DNA fragment containing the _PATROL1_ ORF, obtained by PCR using primers 5′-GACCGGTATGGAAGAAGAAAATGCTGTC-3′ and
5′-GAGCTCCCCGGGGATCGTAAACTACAAACATTTGTA-3′, was inserted into the pGEM-T Easy vector to produce pG-_PATROL1_. The _Sac_II–_Age_I fragment of pG-35S:GFP1 containing the CaMV 35S promoter and
_GFP_ cDNA was inserted into the _Sac_II/_Age_I sites of pG-_PATROL1_ to produce pG-35S:_GFP–PATROL1_. The _Xho_I-_Sma_I fragment of pG-35S:_GFP–PATROL1_ was inserted into the _Xho_I/_Sma_I
sites of pPZP2H-lac. For 35S:_GFP–AHA1_, a DNA fragment containing _AHA1_ ORF was obtained by PCR using primers 5′-TATAAGGGAGGTGGAGGTGCTATGTCAGGTCTCGAAG-3′ and
5′-AGAACTAGTGGATCCCCCGGGTTTTAGAATTAAATTTAAATATTAT-3′. The _Age_I/_Sma_I fragment obtained by digestion of the PCR-amplified _AHA1_ was inserted together with an _Xho_I/_Age_I fragment of
35S:GFP into the _Xho_I/_Sma_I sites of pPZP2H-lac46. For 35S:RFP–AHA1 production, RFP was obtained by PCR using primers 5′-AGGATCCATGGCCTCCTCCGAGGA-3′ and 5′-CATAGCACCTCCACCTCCCTTATA
GGCGCCGGTGGAGTGGCGGC-3′ with mRFP plasmid as a template. A DNA fragment containing AHA1 ORF was obtained by PCR using primers 5′-GCCGCCACTCCACCGGCGCCTATAAGGGAGGTGGAGGTGCT-3′ and
5′-AGAACTAGTGGATCCCCCGGGTTTTAGAATTAAATTTAAATATTAT-3′. A DNA fragment containing RFP–AHA1 was obtained by PCR using primers 5′-AGGATCCATGGCCTCCTCCGAGGA-3′ and
5′-AGAACTAGTGGATCCCCCGGGTTTTAGAATTAAATTTAAATATTAT-3′ with RFP and AHA1 fragments as templates. The _Bam_HI/_Sma_I fragment obtained by digestion of the PCR-amplified RFP–AHA1 was inserted
into _Bam_HI/_Sma_I sites of pPZP2Ha3. A _pPATROL1::GUS_ construct was obtained by amplifying 2 kb of the _PATROL1_ promoter region from genomic DNA using the oligonucleotides
5′-CGGTCGACACAACCACTAGC-3′ and 5′-GGATCCCTCGATCCAGCTGCAATAAT-3′; the product was then inserted into the pGEM-T Easy Vector. A _Sal_I-_Bam_HI fragment including the _PATROL1_ promoter
sequences was cloned into the _Sal_I and _Bam_HI sites of pBI101. IMMUNOHISTOCHEMICAL ANALYSIS Immunohistochemical detection of the plasma membrane H+-ATPase in the epidermis was performed
as described33 with minor modifications. Plants were incubated under low CO2 (<100 p.p.m.) for at least an hour. The abaxial epidermis of the rosette leaves was peeled with a forceps, and
immediately incubated in 4% (_w_/_v_) formaldehyde in fixation buffer for 2.5 h at room temperature. The fixed epidermis was rinsed with water and attached to an MAS-coated glass slide. The
samples were digested by cellulase solutions (1% Cellulase Onozuka R-10 (Yakult) and 0.1% Macerozyme R-10 (Yakult) in PBS) for 20 min at 37 °C. The samples were rinsed twice with PBS and
permeabilized with 0.5% (_w_/_v_) Triton X-100 for 30 min, and then were blocked with 3% bovine serum albumin Fraction V. The samples were incubated with anti-plasma membrane H+-ATPase
(anti-AHA; Cosmo Bio) at a dilution of 1:1,000 for 16 h. The secondary antibody used here was Alexa Fluor 594 goat anti-rabbit IgG (Invitrogen; 1:500) for 3 h in the dark. After washing the
samples, each specimen was mounted on a glass slide with 50% (v/v) glycerol. MICROSCOPY The leaves were covered by a glass slide and observed under a fluorescence microscope (IX70, Olympus)
equipped with an UPlanApo × 100/1.35 oil iris objective lens and a confocal scanning head (CSU10, Yokogawa). For staining endosomes, the seedlings were treated with 33 μM FM4-64
(Invitrogen), for 2 min and then washed with basal buffer (5 mM MES-Tris, 10 mM CaCl2, 50 mM KCl, pH6.5). The hypocotyl epidermis was examined immediately. GFP and FM4-64 were excited with a
laser (HPU-50101-PFS2; Furukawa) at 488 nm and the fluorescence was detected with a cooled CCD camera head system (CoolSNAP HQ, Photometrics) through a 524–546 nm band-pass filter
(FF01-535/22-25, Semrock) for GFP, and a 604–644 band-pass filter (FF01-624/40-25, Semrock) for FM4-64. For light treatment, the leaves were irradiated with 200 μmol m−2 s−1 white light for
30 min with a cold light illumination system (LG-PS2, Olympus). In order to prevent drying, 3-mm-thick 1% agarose gels with basal buffer were placed onto the leaves48. For osmotic
treatments, leaves were incubated in basal buffer (120 mOsM) or basal buffer with 0.4 M Mannitol (530 mOsM) for 15–30 min. Fluorescence from the secondary antibody, Alexa Fluor 594, was
collected using a 561 nm laser (85-YCA-025-040, CVI Melles Griot) for excitation and a 580–600 nm band-pass filter (FF01-590/20-25, Semrock) for emission. For quantification of the Alexa
Fluor 594 intensity, we measured the background-subtracted mean intensity in the signal regions that were segmented with Otsu’s thresholding algorithm49. ISOLATION OF GUARD CELL AND
MESOPHYLL CELL PROTOPLASTS GCPs from 600 to 1,200 leaves were isolated as described25. MCPs were isolated as described elsewhere50 with some modifications. Twenty leaves from a 3–4-week-old
plant were cut into 1-mm strips using a razor blade. Leaf strips were transferred into enzyme solution (5 mM MES (pH 5.6) containing 1% (_w_/_v_) cellulase R-10, 0.4% (_w_/_v_) macerozyme
R-10, 0.1% (_w_/_v_) polyvinylpyrrolidone K-30 (PVP-40), 0.6 M mannitol, 0.5 mM CaCl2, 0.5 mM MgCl2 and 0.2% (_w_/_v_) BSA), and incubated at 24 °C for 2 h. Protoplasts were filtered through
double layers of 50 μm nylon mesh and collected by centrifugation at 140 _g_ for 4 min. GCP and MCP protoplasts were subjected to density gradient centrifugation at 200 _g_ for 20 min using
Histopaque-1077. The purity of the GCPs and MCPs were always higher than 97 and 99%, respectively. TRANSGENE EXPRESSION ANALYSIS Histochemical staining of GUS activity in _pPATROL1::GUS_
transformants was assayed with 5-bromo-4-chloro-3-indolyl-D-glucuronide as a substrate9. To obtain tissue sections, GUS-stained samples were embedded in Technovit 8100 according to the
manufacturers’ instructions (Heraeus Kulzer GmbH, Wehrheim, Germany). A rotary microtome (Yamato Kohki) was used to cut 10-μm-thick sections. Total RNA from plant tissues or protoplasts were
extracted and single-stranded cDNA synthesized from total RNA was used as RT-PCR templates according to the method described by Sugimoto _et al._51. The RT-PCR primers for _PATROL1_ were
5′-CTTCAGAGATATCGCCGGGA-3′ and 5′-CAATCCTGATGAGCTCTGAG-3′. An amplified 700-bp fragment of the _EF1α_ cDNA was used as an internal standard9. Expression of _PATROL1_ in guard cell and
mesophyll cell protoplasts was examined by RT-PCR. The _HT1_ gene was used as a guard-cell-specific expression control9, and the _CBP_ marker gene was used as a mesophyll-cell-specific
expression control52 using primers 5′-CTTATCTGGAGGTGCCACAA-3′ and 5′-CCTCACTCTTTCTTGGATAAC-3′. WATER-LOSS MEASUREMENT The weight of detached rosette leaves was determined every 8 min.
Three-week-old 1/2 MS plate-grown plants were used. Water loss was expressed as the percentage of initial fresh weight. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Hashimoto-Sugimoto,
M. _et al._ A Munc13-like protein in _Arabidopsis_ mediates H+-ATPase translocation that is essential for stomatal responses. _Nat. Commun._ 4:2215 doi: 10.1038/ncomms3215 (2013). REFERENCES
* Willmer, C. M. & Fricker, M. D. _Stomata_ 2nd edn Chapman & Hall: London, (1996). * Hetherington, A. M. & Woodward, F. I. The role of stomata in sensing and driving
environmental change. _Nature_ 424, 901–908 (2003). Article CAS ADS Google Scholar * Kim, T. H., Bohmer, M., Hu, H., Nishimura, N. & Schroeder, J. I. Guard cell signal transduction
network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. _Annu. Rev. Plant Biol._ 61, 561–591 (2010). Article CAS Google Scholar * Schroeder, J. I., Raschke, K. &
Neher, E. Voltage dependence of K+ channels in guard-cell protoplasts. _Proc. Natl Acad. Sci. USA_ 84, 4108–4112 (1987). Article CAS ADS Google Scholar * Kinoshita, T. & Hayashi, Y.
New insights into the regulation of stomatal opening by blue light and plasma membrane H+-ATPase. _Int. Rev. Cell Mol. Biol._ 289, 89–115 (2011). Article CAS Google Scholar * Gaxiola, R.
A., Palmgren, M. G. & Schumacher, K. Plant proton pumps. _FEBS Lett._ 581, 2204–2214 (2007). Article CAS Google Scholar * Merlot, S. et al. Constitutive activation of a plasma
membrane H+-ATPase prevents abscisic acid-mediated stomatal closure. _EMBO J._ 26, 3216–3226 (2007). Article CAS Google Scholar * Merlot, S. et al. Use of infrared thermal imaging to
isolate _Arabidopsis_ mutants defective in stomatal regulation. _Plant J._ 30, 601–609 (2002). Article CAS Google Scholar * Hashimoto, M. et al. _Arabidopsis_ HT1 kinase controls stomatal
movements in response to CO2 . _Nat. Cell Biol._ 8, 391–397 (2006). Article CAS Google Scholar * Richmond, J. E., Davis, W. S. & Jorgensen, E. M. UNC-13 is required for synaptic
vesicle fusion in _C. elegans_. _Nat. Neurosci._ 2, 959–964 (1999). Article CAS Google Scholar * Augustin, I., Rosenmund, C., Südhof, T. C. & Brose, N. Munc13-1 is essential for
fusion competence of glutamatergic synaptic vesicles. _Nature_ 400, 457–461 (1999). Article CAS ADS Google Scholar * Jahn, R. & Scheller, R. H. SNAREs--engines for membrane fusion.
_Nat Rev._ 7, 631–643 (2006). Article CAS Google Scholar * Ma, C., Li, W., Xu, Y. & Rizo, J. Munc13 mediates the transition from the closed syntaxin-Munc18 complex to the SNARE
complex. _Nat. Struct. Mol. Biol._ 18, 542–549 (2011). Article CAS Google Scholar * Basu, J. et al. A minimal domain responsible for Munc13 activity. _Nat. Struct. Mol. Biol._ 12,
1017–1018 (2005). Article CAS Google Scholar * Koch, H., Hofmann, K. & Brose, N. Definition of Munc13-homology-domains and characterization of a novel ubiquitously expressed Munc13
isoform. _Biochem. J._ 349, 247–253 (2000). Article CAS Google Scholar * Pei, J., Ma, C., Rizo, J. & Grishin, N. V. Remote homology between Munc13 MUN domain and vesicle tethering
complexes. _J. Mol. Biol._ 391, 509–517 (2009). Article CAS Google Scholar * Li, W. et al. The crystal structure of a Munc13 C-terminal module exhibits a remarkable similarity to vesicle
tethering factors. _Structure_ 19, 1443–1455 (2011). Article CAS Google Scholar * Schmid, M. et al. A gene expression map of _Arabidopsis thaliana_ development. _Nat. Genet._ 37, 501–506
(2005). Article CAS MathSciNet Google Scholar * Wang, Y. et al. Transcriptome analyses show changes in gene expression to accompany pollen germination and tube growth in _Arabidopsis_.
_Plant Physiol._ 148, 1201–1211 (2008). Article CAS ADS Google Scholar * Bolte, S. et al. FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. _J.
Microsc._ 214, 159–173 (2004). Article CAS MathSciNet Google Scholar * van Gisbergen, P. A., Esseling-Ozdoba, A. & Vos, J. W. Microinjecting FM4-64 validates it as a marker of the
endocytic pathway in plants. _J. Microsc._ 231, 284–290 (2008). Article CAS MathSciNet Google Scholar * Kalla, S. et al. Molecular dynamics of a presynaptic active zone protein studied
in Munc13-1-enhanced yellow fluorescent protein knock-in mutant mice. _J. Neurosci._ 26, 13054–13066 (2006). Article CAS Google Scholar * Ueno, K., Kinoshita, T., Inoue, S., Emi, T. &
Shimazaki, K. Biochemical characterization of plasma membrane H+-ATPase activation in guard cell protoplasts of _Arabidopsis thaliana_ in response to blue light. _Plant. Cell Physiol._ 46,
955–963 (2005). Article CAS Google Scholar * Marmagne, A. et al. Identification of new intrinsic proteins in _Arabidopsis_ plasma membrane proteome. _Mol. Cell. Proteomics_ 3, 675–691
(2004). Article CAS Google Scholar * Negi, J. et al. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. _Nature_ 452, 483–486 (2008). Article CAS
ADS Google Scholar * Vahisalu, T. et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. _Nature_ 452, 487–491 (2008). Article CAS ADS
Google Scholar * Zhao, Z., Zhang, W., Stanley, B. A. & Assmann, S. M. Functional proteomics of _Arabidopsis thaliana_ guard cells uncovers new stomatal signaling pathways. _Plant Cell_
20, 3210–3226 (2008). Article CAS Google Scholar * Cutler, S. R., Ehrhardt, D. W., Griffitts, J. S. & Somerville, C. R. Random GFP::cDNA fusions enable visualization of subcellular
structures in cells of _Arabidopsis_ at a high frequency. _Proc. Natl Acad. Sci USA_ 97, 3718–3723 (2000). Article CAS ADS Google Scholar * Hedrich, R. Ion channels in plants. _Physiol.
Rev._ 92, 1777–1811 (2012). Article CAS ADS Google Scholar * Eisenach, C., Chen, Z. H., Grefen, C. & Blatt, M. R. The trafficking protein SYP121 of Arabidopsis connects programmed
stomatal closure and K+ channel activity with vegetative growth. _Plant J._ 69, 241–251 (2011). Article Google Scholar * Lisenbee, C. S., Karnik, S. K. & Trelease, R. N. Overexpression
and mislocalization of a tail-anchored GFP redefines the identity of peroxisomal ER. _Traffic_ 4, 491–501 (2003). Article CAS Google Scholar * Campbell, R. E. et al. A monomeric red
fluorescent protein. _Proc. Natl Acad. Sci USA_ 99, 7877–7882 (2002). Article CAS ADS Google Scholar * Hayashi, M., Inoue, S., Takahashi, K. & Kinoshita, T. Immunohistochemical
detection of blue light-induced phosphorylation of the plasma membrane H+-ATPase in stomatal guard cells. _Plant Cell Physiol._ 52, 1238–1248 (2011). Article CAS Google Scholar * Turner,
N. C. & Graniti, A. Fusicoccin: a fungal toxin that opens stomata. _Nature_ 223, 1070–1071 (1969). Article CAS ADS Google Scholar * Léon-Kloosterziel, K. M. et al. Isolation and
characterization of abscisic acid-deficient _Arabidopsis_ mutants at two new loci. _Plant J._ 10, 655–661 (1996). Article Google Scholar * Südhof, T. C. The presynaptic active zone.
_Neuron_ 75, 11–25 (2012). Article Google Scholar * Homann, U. & Thiel, G. Unitary exocytotic and endcytotic events in guard-cell protoplasts during osmotically driven volume changes.
_FEBS Lett._ 460, 495–499 (1999). Article CAS Google Scholar * Shope, J. C., DeWald, D. B. & Mott, K. A. Changes in surface area of intact guard cells are correlated with membrane
internalization. _Plant Physiol._ 133, 1314–1321 (2003). Article CAS Google Scholar * Grefen, C., Honsbein, A. & Blatt, M. R. Ion transport, membrane traffic and cellular volume
control. _Curr. Opin. Plant Biol._ 14, 332–339 (2011). Article CAS Google Scholar * Besserer, A. et al. Selective regulation of Maize plasma membrane aquaporin trafficking and activity by
the SNARE SYP121. _Plant Cell_ 24, 3463–3481 (2012). Article CAS Google Scholar * Paciorek, T. et al. Auxin inhibits endocytosis and promotes its own efflux from cells. _Nature_ 435,
1251–1256 (2005). Article CAS ADS Google Scholar * Hager, A. Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: historical and new aspects. _J. Plant Res._ 116,
483–505 (2003). Article CAS Google Scholar * Gévaudant, F. et al. Expression of a constitutively activated plasma membrane H+-ATPase alters plant development and increases salt tolerance.
_Plant Physiol._ 144, 1763–1776 (2007). Article Google Scholar * Haruta, M. et al. Molecular characterization of mutant _Arabidopsis_ plants with reduced plasma membrane proton pump
activity. _J. Biol. Chem._ 285, 17918–17929 (2010). Article CAS Google Scholar * Monda, K. et al. Environmental regulation of stomatal response in the _Arabidopsis_ Cvi-0 ecotype.
_Planta_ 234, 555–563 (2011). Article CAS Google Scholar * Fuse, T., Sasaki, T. & Yano, M. Ti-plasmid vectors useful for functional analysis of rice genes. _Plant Biotechnol._ 18,
219–222 (2001). Article CAS Google Scholar * Sugimoto, H. et al. The rice nuclear gene, _VIRESCENT 2_, is essential for chloroplast development and encodes a novel type of guanylate
kinase targeted to plastids and mitochondria. _Plant J._ 52, 512–527 (2007). Article CAS Google Scholar * Higaki, T. et al. Statistical organelle dissection of _Arabidopsis_ guard cells
using image database LIPS. _Sci. Rep._ 2, 405 (2012). Article Google Scholar * Otsu, N. A threshold selection method from gray-level histograms. _IEEE Trans. Sys. Man. Cyber._ 9, 62–66
(1979). Article Google Scholar * Yoo, S.-D., Cho, Y.-H. & Sheen, J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. _Nat. Protoc._ 2,
1565–1572 (2007). Article CAS Google Scholar * Sugimoto, H. et al. The _virescent-2_ mutation inhibits translation of plastid transcripts for the plastid genetic system at an early stage
of chloroplast differentiation. _Plant Cell Physiol._ 45, 985–996 (2004). Article CAS Google Scholar * Mori, I. C. et al. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell
S-type anion- and Ca2+-permeable channels and stomatal closure. _PLoS Biol._ 4, e327 (2006). Article Google Scholar Download references ACKNOWLEDGEMENTS We are grateful to N. Brose and L.
Serna for critical reading of the manuscript, and E.M. Jorgensen, J.I. Schroeder and T. Teramoto for comments on the manuscript. We thank T. Sakaguchi for the technical assistance. We also
thank R.Y. Tsien for making the mRFP gene available, and the Arabidopsis Biological Resource Center and Cereon Genomics for access to polymorphism information. This research was supported in
part by Grants-in-Aid for 21114002 (K.I.), 24114007 (S.H.), 22114505 (S.H), 24228008 (K.S.), 25711017 (T.H.) and 24780046 (T.Y.) from the Ministry of Education, Science and Culture of
Japan, and by the Program for Promotion of Basic and Applied Research for Innovations in Bio-Oriented Industry (K.I.), and the Advanced Measurement and Analysis grant from the Japan Science
and Technology Agency (S.H.). M.H.-S. is grateful for the financial support from the Fumi Yamamura Memorial Foundation for Female Natural Scientists. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Department of Biology, Faculty of Sciences, Kyushu University, Fukuoka, 812-8581, Japan Mimi Hashimoto-Sugimoto, Ayako Nagami, Mari Irie, Miho Fujimi, Megumi Miyamoto, Juntaro
Negi & Koh Iba * Department of Integrated Biosciences, Graduate School of Frontier Sciences, The University of Tokyo, Kashiwanoha Kashiwa, 277-8562, Chiba, Japan Takumi Higaki, Kae
Akita & Seiichiro Hasezawa * Department of Advanced Measurement and Analysis, Japan Science and Technology Agency (JST), Chiyoda-ku, 102-0076, Tokyo, Japan Takumi Higaki & Seiichiro
Hasezawa * Plant Science Center, RIKEN, 1-7-22 Suehiro-cho, Tsurumi, Yokohama, 230-0045, Kanagawa, Japan Takashi Yaeno & Ken Shirasu Authors * Mimi Hashimoto-Sugimoto View author
publications You can also search for this author inPubMed Google Scholar * Takumi Higaki View author publications You can also search for this author inPubMed Google Scholar * Takashi Yaeno
View author publications You can also search for this author inPubMed Google Scholar * Ayako Nagami View author publications You can also search for this author inPubMed Google Scholar *
Mari Irie View author publications You can also search for this author inPubMed Google Scholar * Miho Fujimi View author publications You can also search for this author inPubMed Google
Scholar * Megumi Miyamoto View author publications You can also search for this author inPubMed Google Scholar * Kae Akita View author publications You can also search for this author
inPubMed Google Scholar * Juntaro Negi View author publications You can also search for this author inPubMed Google Scholar * Ken Shirasu View author publications You can also search for
this author inPubMed Google Scholar * Seiichiro Hasezawa View author publications You can also search for this author inPubMed Google Scholar * Koh Iba View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS M.H.-S. and K.I. designed the studies and wrote the paper. M.H.-S. performed most of the experiments together with A.N., M.I.,
M.F., M.M., and J.N.; T.H., K.A. and S.H. performed imaging analysis with fluorescence microscope. T.Y. and K.S. measured fresh weight of the plants grown under short days. All authors
discussed the results and commented on the manuscript. CORRESPONDING AUTHOR Correspondence to Koh Iba. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial
interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figures S1-S8 (PDF 15838 kb) RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons
Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/ Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Hashimoto-Sugimoto, M., Higaki, T., Yaeno, T. _et al._ A Munc13-like protein in _Arabidopsis_ mediates H+-ATPase translocation that is essential for stomatal responses.
_Nat Commun_ 4, 2215 (2013). https://doi.org/10.1038/ncomms3215 Download citation * Received: 29 May 2013 * Accepted: 02 July 2013 * Published: 30 July 2013 * DOI:
https://doi.org/10.1038/ncomms3215 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative