- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Achieving significant adhesion to soft tissues while minimizing tissue damage poses a considerable clinical challenge. Chemical-based adhesives require tissue-specific reactive chemistry,
typically inducing a significant inflammatory response. Staples are fraught with limitations including high-localized tissue stress and increased risk of infection, and nerve and blood
vessel damage. Here inspired by the endoparasite Pomphorhynchus laevis, which swells its proboscis to attach to its host’s intestinal wall, we have developed a biphasic microneedle array
that mechanically interlocks with tissue through swellable microneedle tips, achieving ~3.5-fold increase in adhesion strength compared with staples in skin graft fixation, and removal force
of ~4.5 N cm−2 from intestinal mucosal tissue. Comprising a poly(styrene)-block-poly(acrylic acid) swellable tip and non-swellable polystyrene core, conical microneedles penetrate tissue
with minimal insertion force and depth, yet high adhesion strength in their swollen state. Uniquely, this design provides universal soft tissue adhesion with minimal damage, less traumatic
removal, reduced risk of infection and delivery of bioactive therapeutics.
Achieving significant levels of adhesion to soft tissues presents considerable challenges, especially when tissues are wet1,2. Chemical adhesives, such as cyanoacrylate, adhere strongly to
tissues by a reactive exothermic covalent crosslinking reaction. However, these adhesives often cure too fast or too slowly and release toxic degradation products such as formaldehyde,
leading to an intense inflammatory response3. While clinically utilized fibrin glue can effectively bond to wet tissue without a significant inflammatory response, it exhibits low strength
of adhesion due to poor cohesive properties4, and can be challenging to apply as the polymerization time is difficult to control during placement. Alternatively, biocompatible hydrogel
adhesives5,6 that covalently bond to specific tissues can achieve a significant level of adhesion, yet their effectiveness depends on the presence of surface biomolecules with specific
functional groups (e.g., NH2, SH or COOH). Therefore, the chemistry must be tailored for each tissue, and hydrogel adhesives do not adhere strongly to tissues such as skin. Also, it is
critical to note that adhesives that rely on chemical bonding can easily be fouled in the presence of blood, thus compromising their efficacy in many surgical settings. For universal use,
gold standard sutures and staples based on mechanical fixation are widely used7,8,9, however their placement can extend operating times as the tissue often must be manipulated before each
pass of a suture needle. Sutures are difficult to place in small spaces (that is, during laparoscopic procedures)10, and do not work effectively for repair of many tissues including the dura
mater7, urethral defects8 and lung tissue9. While staples are typically quicker and easier to place than sutures, they can cause significant tissue damage, scarring, and excessive depth of
tissue penetration can result in nerve and blood vessel damage11. Staples also pose a higher risk of developing a wound infection compared with sutures12. There are several unmet clinical
needs for adhesives to affix connective tissues, including tendons and ligaments, to improve contact between tissues to reduce motion of tissue grafts, and to seal tissues for prevention of
fluid (intestine) or air (lung) leaks. New adhesives are also needed to prevent formation of seromas following operations that create dead space between layers of tissue (for example,
abdominoplasty), thus avoiding the need for drains that increase the risk of infection and potential necessity for reoperation13.
A suitable platform approach to overcome the limitations of existing adhesives should ideally avoid the use of reactive chemistry, provide strong tissue adhesion in both normal and shear
directions, be amenable to quick application, be simple to position over the target site, be removable (or degradable) with minimal tissue damage, provide adhesion to dynamic tissue surfaces
while withstanding multiple extension/compression cycles, minimize the risk of infection and have the capacity to deliver therapeutics. Compared with glues, sutures and staples, tissue
adhesive tapes have shown significant advantages including reduced procedure times, reduced scarring, ability to spread tissue forces over a larger surface area and enhanced tissue
handling14,15,16. Additionally, adhesive tapes may be useful as an internal equivalent of a transdermal drug delivery patch to deliver drugs such as antibacterial, proregenerative or
anti-inflammatory agents to target tissues. While barrier membranes and hernia meshes are routinely used clinically, these typically provide minimal adhesion, and often several sutures or
tacks are used for fixation. Tape adhesives with hook-like protrusions have been developed, however their adhesion to tissue is fundamentally limited to surface entanglement without
penetration of the tissue17.
In seeking an optimal method of universal soft tissue adhesion with minimal tissue damage, we looked for inspiration from living organisms that have through the course of evolution adapted
this function. Endoparasitic worms known as spiny-headed worms use a proboscis to penetrate through tissue. Species, such as Pomphorhynchus laevis, secure firm anchorage to the fish
intestinal wall by expanding a bulb using retractor muscles at the base of the proboscis following penetration18. Using the adaptable morphology of the worm proboscis, we looked to create a
structured biphasic microneedle (MN) with optimal characteristics for needle insertion and retention in tissue.
Here we demonstrate a highly engineered MN with a shape change swellable tip that facilitates mechanical interlocking with tissue (Fig. 1a). This design minimizes the force needed for tissue
penetration, as the smooth, cone-shaped needles can be inserted into tissue in a dry (stiff) state and do not include protruding barb features, associated with other proposed MN-based
adhesive platforms19. Significant pull-out force can be achieved through the rapid increased cross-sectional area that occurs with swelling at the needle tips on contact with water (in
tissue), leading to localized tissue deformation and subsequent interlocking. The bio-inspired MN adhesive showed high levels of adhesion with wet tissues, such as skin and intestine tissue,
regardless of differences in surface texture. Additionally, strong fixation can be achieved in dynamic tissues during multiple cycles of movement and as increased pull-out forces are
achieved via swelling within tissue, the soft MN tips (that is, reduced modulus) enable removal without significantly damaging the tissue. Furthermore, unlike stiff MNs that may break during
removal from tissue, the modulus change due to swelling prevents breakage of the swollen MNs.
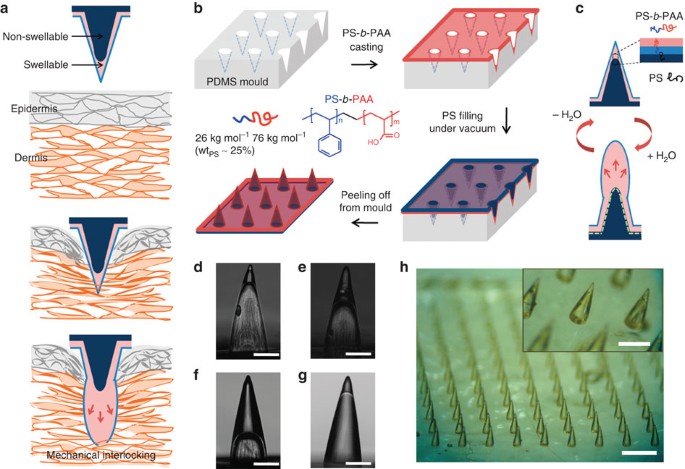
(a) Illustration showing mechanical interlocking of a water-responsive shape-changeable microneedle following penetration into a tissue. (b) Schematic showing the preparation of the
double-layered MN array using a PDMS mould and showing the chemical structure of PS-b-PAA with PS weight fraction (wtPS) of 25% used for the swellable tip. (c) Cartoon showing the inner
structure of double-layered MN and reversible water responsiveness. A thin hydrophobic film comprising the PS block covered the outer surface of the MN, likely due to the presence of the
hydrophobic PDMS mould. During filling of the non-swellable PS core, PS chains likely entangle with the PS block at the interface (dashed line) between the swellable tip and PS core,
providing interfacial adhesion to prevent delamination. (d–f) Side view images of hollow MN (without the PS core) with different PS-b-PAA filling fractions, including (d) 20%, (e) 40% and
(f) 70% (height of PS-b-PAA layer compared with total MN height). These MNs were prepared by solvent-casting PS-b-PAA dissolved in DMF using different concentrations (d: 10 wt%, e: 18 wt%
and f: 25 wt%). (g) Double-layered MN with a swellable tip (20% height fraction) following filling of the PS core. Scale bar, 200 μm. (h) Photograph of the double-layered MN array with
density of 10 × 10 cm−2 showing high-pattern fidelity. Scale bar, 1 mm. The PS-b-PAA tip is clearly distinguishable from the PS core as shown in the inset (Scale bar, 500 μm).
To create a stimulus responsive MN platform with low penetration force and strong adhesion, we pursued a double-layered MN design with selective localization of swellable material in the tip
region. Following preferential distal swelling, each MN had both soft (outer) and stiff (inner) regions. In addition to having a MN platform comprising both swellable and non-swellable
components, we sought to control the mechanical and water permeation properties of the swellable material, while promoting significant interaction between the soft outer layer and the stiff
inner layer to prevent delamination. For this purpose, we considered an amphiphilic block copolymer (BCP) design that exhibits selective responsiveness to stimuli such as the presence of
aqueous or organic solvents20,21. We envisioned that dual hydrophilic–hydrophobic properties of the outer layer would enable rapid absorption of water and promote intimate interaction with
the non-swellable inner hydrophobic region. Block copolymers contain two or more chemically different polymers connected by covalent bonds that offer a means of combining the desirable
characteristics of different polymers into a hybrid material22. The mechanical properties and swellability of the outer region of the MNs can be controlled by manipulating the overall
average molecular weight of the polymer and the weight fraction of each block20,23. In this study, we chose a polystyrene-block-poly(acrylic acid) (PS-b-PAA) block copolymer as the swellable
material and PS homopolymer as a non-swellable material. PAA is a well-known super-absorbent polymer used in several biomaterials-based strategies and in consumer products such as diapers
that possesses COOH groups that quickly become ionized in the presence of water. In contrast, PS exhibits mechanical strength and structural integrity without swelling. As predicted, high
volume expansion occurred when PAA was the major block component (over 70% in weight fraction). The mechanical robustness of the block copolymer could be strengthened by increasing the
weight fraction of the PS block (Supplementary Fig. S1). Interestingly, the stiffness of the block copolymer in its swollen state, with PS of ~25% weight fraction, approximates that of skin
or intestine tissue (Supplementary Fig. S1), which reduces the risk of underlying tissue damage without significantly compromising the interlocking mechanism. To permit rapid water
penetration into the PS-b-PAA layer when the PS block preferentially assembles at the air-copolymer surface, we selected a PS block with a relatively low-molecular weight (


:max_bytes(150000):strip_icc():focal(749x0:751x2)/texas-school-shooting-74-fc33ee8f509244eeb184bcd7d551841b.jpg)




