- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Despite decades of cognitive, neuropsychological and neuroimaging studies, it is unclear if letters are identified before word-form encoding during reading, or if letters and their
combinations are encoded simultaneously and interactively. Here using functional magnetic resonance imaging, we show that a ‘letter-form’ area (responding more to consonant strings than
false fonts) can be distinguished from an immediately anterior ‘visual word-form area’ in ventral occipito-temporal cortex (responding more to words than consonant strings). Letter-selective
magnetoencephalographic responses begin in the letter-form area ∼60 ms earlier than word-selective responses in the word-form area. Local field potentials confirm the latency and location
of letter-selective responses. This area shows increased high-gamma power for ∼400 ms, and strong phase-locking with more anterior areas supporting lexico-semantic processing. These findings
suggest that during reading, visual stimuli are first encoded as letters before their combinations are encoded as words. Activity then rapidly spreads anteriorly, and the entire network is
engaged in sustained integrative processing. You have full access to this article via your institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS SPATIOTEMPORAL DYNAMICS OF
ORTHOGRAPHIC AND LEXICAL PROCESSING IN THE VENTRAL VISUAL PATHWAY Article 30 November 2020 BINDING OF CORTICAL FUNCTIONAL MODULES BY SYNCHRONOUS HIGH-FREQUENCY OSCILLATIONS Article 12 August
2024 THE DYNAMICS OF READING COMPLEX WORDS: EVIDENCE FROM STEADY-STATE VISUAL EVOKED POTENTIALS Article Open access 05 August 2021 INTRODUCTION Fluent readers distinguish between thousands
of subtly different visual stimuli, associating each with a different meaning within a few hundred milliseconds. Some models of reading suppose that visual stimuli are identified as letters
before their ordered combinations are identified as words, noting that brain lesions can specifically impair the ability to recognize letters1, or to identify single letters but not whole
words2. Such cases are countered by studies in healthy subjects showing that letters are more quickly and accurately identified within the context of words (the ‘word superiority effect’),
suggesting that letter- and word-recognition may not be sequential and separable, but rather simultaneous and integrated3. More recently, neuroimaging studies have identified a ‘visual
word-form area’ (VWFA), showing increased hemodynamic activation to words compared with sensory controls, and centred in the left posterior fusiform gyrus (lpFg; for review see 4, for
limitations to this concept see 5). Critically, activation in this area to letter-strings increases with their similarity to actual words6,7, especially in more anterior VWFA8, suggested
that it actually comprises a succession of detectors responding to progressively more abstract lexico-semantic aspects of the letter-strings. A word-selective response can also be recorded
with Electroencephalography (EEG), peaking over the left occipital scalp at ∼140–220 ms9. This response has been localized to lpFg with magnetoencephalography (MEG)10,11 and intracranial
local field potentials (LFP)12,13,14. In contrast to the strong multimodal evidence for word-form processing in VWFA, the evidence for separable letter-form processing is equivocal. Although
several studies have reported larger EEG responses to letter-strings as compared with false fonts (FF) over left lateral occipital scalp, it is not clear if these differ in either latency
or location from word-form responses9,15. Functional magnetic resonance imaging (fMRI) provides more certain localization, but has not identified areas where letter-strings reliably evoke
more activity than FF within lpFg, nor has it been able to provide information regarding the timing of these processes8,16. Here we identify a putative letter-form area immediately posterior
to the VWFA with fMRI in healthy subjects, and show with MEG that letter-selective activation estimated to the putative letter-form area precedes the word-selective activation in the VWFA.
Next, we use LFP recorded directly from the letter-form area using pial electrodes in epileptic patients to confirm and extend the non-invasive measures, providing converging evidence for a
separate letter-form area preceding in time and anatomy of the VWFA. Finally, we show using intracranial recordings that activation of the putative letter-form area is prolonged, overlapping
and phase-locked with anterior language areas during later, but not earlier, stages of reading. RESULTS LETTER- AND WORD-SELECTIVITY We recorded brain activity in English readers evoked by
FF arranged in a string like a word, by consonant strings (CS), and by real words (RW). We reasoned that if separate letter-form and word-form processing stages exist, they would be indexed
by CS>FF, and RW>CS contrasts, respectively. Stimuli were presented every 600 ms with no gap, and the subject responded to rare (<5%) animal names. This task required the subject to
attempt to read each stimulus, the cognitive process under examination. Although non-word stimuli would thus be subjected to less processing once they were identified as such, our main
focus was on the first pass of neural activity occurring before definitive word identification. HEMODYNAMIC RESPONSES First, we used fMRI in 12 healthy subjects to isolate candidate areas in
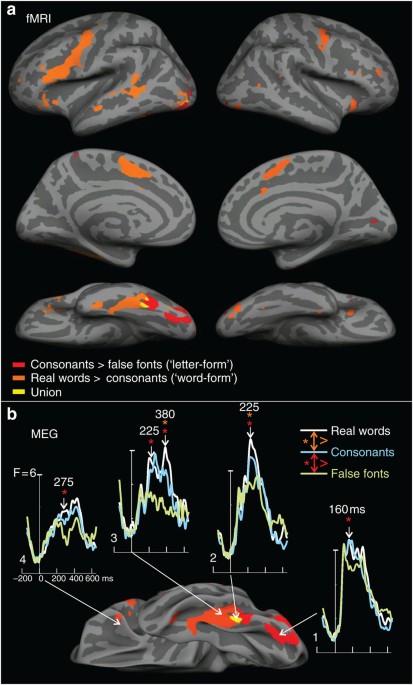
lpFg. Letter-selective (CS>FF) hemodynamic activation was restricted to lpFg, and word-selective (RW>CS) processing was immediately anterior, with very little overlap (Figs 1a and 2).
Word-selective areas extended beyond the lpFg to traditional language areas (Wernicke’s and Broca’s), as well as cingulate gyrus and contralateral sites. In order to maximize single subject
signal-to-noise-ratio (SNR) we used a block design for the fMRI modality only. Thus the subjects may have used shallower processing for the non-word stimuli, accentuating their difference
from words. Furthermore, the contrast RW>CS would be expected to reveal areas processing more abstract lexical and semantic properties, as well as those processing word-forms.
Nonetheless, the fMRI study accomplished its goal, to localize for further study candidate structures in lpFg that might underlie letter-form and word-form processing.
MAGNETOENCEPHALOGRAPHIC RESPONSES Owing to the nature of neurovascular coupling, hemodynamic measures cannot distinguish the onsets of neural processing stages that differ by less than about
a second. Consequently, we turned to the millisecond accuracy of MEG to examine the time-course of processing evoked by FF, CS and RW within the regions identified by fMRI in the lpFg. By
using a random stimulus order, and concentrating on first-pass processing, we were able to determine when CS>FF and RW>CS effects initially occur, before potentially confounding
effects of differential processing, which could occur only after stimulus identification. MEG is mainly generated by currents within apical dendrites of cortical pyramidal cells. Currents
were estimated with noise-normalized minimum norm constrained by each subject’s MRI17. At 160 ms, the first letter-, but not word-selective differences peak in lpFg (Fig. 1b, area 1).
Word-selective activation emerges later, peaking at 225 ms in an immediately anterior location (Fig. 1b, area 2). At this latency, letter-selective responses are also estimated to this area.
Thus, like hemodynamic activation, the earliest neural currents that were letter-selective but not word-selective were estimated to occur only in the most posterior part of lpFg.
Furthermore, these letter-selective currents peaked earlier than more anterior word-selective responses. Unlike its hemodynamic response, currents in anterior fusiform gyrus showed
letter-selective, as well as word-selective responses (Fig. 1b, area 3). Dissociations between MEG and fMRI may occur because they are sensitive to different aspects of neural activity, and
fMRI integrates activity over a longer time period18. Nonetheless, MEG confirms a succession in time and space of neural currents distinguishing first letters and then words from their
respective controls, confirming the spatial succession shown by hemodynamic measures (Fig. 3). INTRACRANIAL EEG RESPONSES Although providing excellent timing, localizations of MEG generators
are always subject to some uncertainty. Unambiguous localization was obtained with LFP recordings from the lpFg surface using electrodes implanted in epileptic patients for the clinical
purpose of localizing seizure onset relative to eloquent cortex. Nine patients had electrodes located in the ventral occipito-temporal region of the language dominant hemisphere, and had
normal verbal intelligence testing and reading ability (Supplementary Table S1). Electrode contacts considered for analysis were within 1 cm of the group hemodynamic response, were distant
from the ultimately determined seizure focus and from brain abnormalities identified with structural imaging, and had normal-appearing background activity with few or no epileptiform spikes
or slow waves. Of 34 such contacts, 25 recorded LFP (intracranial event-related potential (ERP)) that responded during the task compared with prestimulus baseline. Of these 25 responsive
contacts, 14 responded differentially to CS versus FF before 300 ms (Fig. 4). As the LFP records essentially the same signal locally that the MEG records at a distance, the LFP responses
directly confirm the inferred localization of MEG generators (Supplementary Fig. S1). HIGH-GAMMA BAND POWER The polarity of MEG or LFP does not reliably indicate if the underlying population
is producing increased or decreased neuronal activity. Such information can be derived from broadband high-gamma power (HGP), which arises from summated fast post-synaptic membrane currents
and action potentials. The nine patients were implanted with a total of 1,351 electrodes of which 107 (7.9%) contacts exhibited significant task-related HGP. Of these 107, 7 (6.5%) contacts
recorded greater activation to CS than FF before 250 ms, of which 6 (85%) were in lpFg, thus providing additional evidence that letter-selective activation is mainly localized to this area.
COMMON RESPONSE PATTERNS ACROSS BRAIN-IMAGING MODALITIES The locations and timing of the LFP and HGP responses to words, CS and FF directly recorded from lpFg in patients thus showed a good
correspondence to the fMRI and MEG contrasts recorded from healthy controls. In addition, excellent correspondence was observed in one patient studied with fMRI before electrode
implantation (Fig. 4a), and in another patient studied with both fMRI and MEG recordings (Fig. 4b). The recording electrode on the cortical location showing CS>FF hemodynamic activation
also recorded focal CS>FF LFP and HGP. The HGP response significantly differentiates between CS and FF beginning at ∼140–170 ms, very close to that observed with MEG in the same subject.
The LFP and HGP responses were in most cases highly focal, being absent in the adjacent contacts separated by 6 mm (Fig. 4e). NUMBER OF LETTERS Previous studies have found that the number of
letters does not affect hemodynamic activation of the VWFA, but does affect the immediately posterior region19,20. We also found that the letter-selective HGP responses increased linearly
with the number of letters (Fig. 4f). Specifically, in the two subjects with the highest SNR recordings, the average HGP from 200–300 ms correlated with number of letters in CS (Pearson’s
_r_=0.96, 0.95; both _P_<0.01) and words (Pearson’s _r_=0.94, 0.88; both _P_<0.05) but not FF (_r_=−0.32, 0.64; both _P_>0.2; please see Supplementary Materials for details). Thus,
this correlation with number of letters does not reflect greater sensory stimulation (as it was not seen with increasing numbers of FF stimuli), and is independent of word frequency or
meaning (as CS have neither). When considering the words only, there is no significant correlation with word frequency if the effects of word length are removed (Supplementary Materials),
unlike what has been reported for the VWFA21. These findings show that the processing devoted by the letter-selective area to a stimulus is proportional to the number of letters it contains
but is not sensitive to basic lexical properties such as frequency. These characteristics are consistent with its putative role in processing individual letters instead of whole words, and
distinguish it from the VWFA. TEMPORAL DYNAMICS OF HGP As HGP is highly correlated with hemodynamic activation22, the HGP responses recorded at the location of hemodynamic responses should
indicate the time-course of the neural activity underlying the hemodynamic activations. In the highest SNR HGP recordings, letter-selective activity began at ∼150 ms after CS onset, peaked
at ∼200 ms and continued for over 400 ms (Fig. 4b). Thus, although activation of the putative letter-form area begins before more anterior language areas, it is prolonged and overlaps with
word-form, lexical and lexico-semantic processing. TEMPORAL DYNAMICS OF COMMUNICATION BETWEEN BRAIN REGIONS In order to obtain additional evidence regarding whether these coactivated areas
are communicating, the phase-locking value (PLV) was calculated between active sites23. PLV measures the consistency of the relative phase of LFPs in two locations. High PLV indicates
consistent synchronization of the synaptic currents in pyramidal apical dendrites between the cortical locations underlying the intracranial sensors. Such inferences are weakened in EEG or
MEG by the fact that any two sensors will often record activity from the same cortical location, resulting in spurious correlations24. Intracranial LFP are focally sensitive to the
underlying cortex and thus are not prone to this confound. PLV was strongly elevated during word processing from ∼170–400 ms between the lpFg sites showing letter-selectivity and other
locations responding to words (Fig. 5). In order to test the generality of this finding, a single-trial estimate of the PLV (PLVi) was calculated for 24 electrode-pairs, each between an lpFg
electrode with early CS>FF HGP activation, and another location with temporally overlapping statistically significant differential HGP responses in the same task. Fourteen (58%) showed
significantly increased PLVi (8–35 Hz; 140–300 ms) to words as compared with FF (_P_<0.01; please see Supplementary Materials for details). Although the PLV indicated very-high levels of
phase synchrony during the critical period while reading words, it was at chance levels before word onset, or in response to FF (Fig. 5). Resting-state fMRI correlations have been reported
between the VWFA and other language-related regions25, but other studies have given apparently contradictory results26. In any case, the phase-locking reported here is transient and
restricted to reading, and occurs at an about one thousand times higher frequency (8–35 Hz for PLV as compared with 01–1 Hz for resting-state fMRI correlations), rendering direct comparisons
problematic. The high PLV between the putative letter-form area and anterior language-related areas suggests that although early processing of the visual word during reading is sequential
and modular, later processing is simultaneous and interactive across a widespread network of structures with complementary specializations. Participation by letter-selective regions in the
broader language network is also implied by the picture naming deficits induced by electrical stimulation of the contacts recording letter-selective responses in one subject (Fig. 4j).
DISCUSSION This study replicated previous studies showing word-selective hemodynamic activation in lpFg4, and then demonstrated letter-selective activation in the posteriorly adjacent area.
Previous studies recording the hemodynamic response to CS and FF have either not directly compared them27, not reported their comparison28, found no differences in the lpFg16 or found only
locations with FF>CS8. In most cases, these studies used low-level tasks in order to prevent the possible confound of differential stimulus processing, but this may have unintentionally
biased them against specific letter- or word-form processing. We used a high-level task that required reading for meaning and were able to avoid the possible confound by concentrating on
first-pass processing probed with high-temporal resolution electromagnetic techniques. Owing to the random stimulus order each stimulus could be a word, and thus had to be processed
initially as if it were a word. Eventually, FF were identified as such, attenuating further lexico-semantic processing. However, identification of the stimulus as FF must have occurred after
the stage of interest because the stage of interest is exactly that which performs such identification. Owing to the high-temporal resolution of MEG and electrocorticography (ECoG), we
observed the activity of each stage without contamination by other stages, and distinguished which anatomical location selectively responded to CS versus FF at the shortest latency, even
though many structures eventually showed such effects due to both feedforward and feedback influences at longer latencies. It is possible that FF could have been determined very rapidly to
not be letters and this resulted in fewer resources being devoted to their further processing. Similarly, CS may have been rapidly determined to have no vowels, and thus evoked shallower
processing than RW. If so, it is possible that our measure of CS processing (CS minus FF) was incomplete, for example, in that not all letters were identified during this shallow processing.
However, we note that our task, which requires reading for meaning, is more likely to encourage letter identification than the perceptual tasks, which strive for identical processing of FF,
CS and RW. Indeed, activation by CS of the putative letter-form area was proportional to the number of letters in the string, suggesting that all letters were processed. Finally, even if
the letters in CS were not completely processed in our task (that is, as much as letters in RW), the result would be to decrease the effect size that we observed, not change their
interpretation. Several studies have compared responses with letters versus symbols, sometimes finding greater fMRI activation in lpFg with consistent EEG responses29. Using a low-level
task, Vartiainen _et al._30 did not detect greater fMRI activation to words or letters than to symbols, and other controls in lpFg, but were able to fit dipoles with greater activity to
letters in lateral temporo-occipital cortex. Other studies have found that this area may show fMRI activation with attention31 or working memory32 for single letters as compared with
symbols. Differential MEG activity to symbols has also been localized at early latencies to more postero-medial occipital areas10. This may correspond to the most posterior lpFg differential
fMRI activation noted in the current study (Fig. 1b). A previous intracranial study failed to find any difference in HGP or LFP evoked by FF compared with CS14. However, this study also
used a perceptual task, and sampled the sulci surrounding lpFg with depth electrodes. We recorded from the ventral surface of the lpFg, where the responses were highly focal. Additional
studies are needed to determine if the letter-form area requires a reading task for full activation, and if it extends anatomically from the crown of the lpFg into the surrounding sulci.
Additional studies are also needed to determine if this area responds to stimuli besides letters and words. Using the excellent temporal resolution of MEG we found that the letter-selective
activation in lpFg precedes the more anterior word-selective activity. We confirmed the timing and anatomical location of the letter-form responses identified with the non-invasive measures
with direct intracranial recordings of LFP and HG, and further demonstrated that these responses comprise increased synaptic processing. Our finding that letter-form and word-form processing
are arranged sequentially in the lpFg is consistent with previous studies of reading showing relatively greater activation to higher order lexical and ultimately semantic stimulus
properties in more anterior locations in humans with fMRI8,33, and MEG34,35. Intracranial recordings confirm that the first sweep of activation along the ventral stream extends to Broca’s
region14,36, and comprises a current sink in layer IV with sharply increased firing37. In the anteroventral temporal lobe, first-pass activity to words may even be selective for the semantic
category of the word38. These findings are also consistent with the general posterior-to-anterior gradient in the complexity of visual stimulus processing in the ventral stream demonstrated
with single-unit recordings in monkeys39. Neural activity in the putative letter-form area remained strongly elevated during reading for hundreds of millseconds following the initial
letter-selective activation. This later processing could be sensitive to multiple constraints, and preceded the behavioural response. Furthermore, during these later stages, widely
distributed areas were activated to words, and their activity became strongly but transiently phase-locked with the lpFg electrodes showing early letter-form responses, especially when
reading words. These results resemble the transient phase-locking that occurs between the fusiform face area and more anterior sites in the right hemisphere40, adding to the many parallels,
which have been found between face and word recognition4,41,42. Thus, following the initial feedforward sweep, the current HGP and PLV results strongly support a sustained and interactive
co-activation of a network of sites contributing to reading. This could provide the substrate for distributed calculation of word identity and meaning5, an interpretation that is supported
by the disruption of naming by stimulation of the putative letter-form area in one patient. The top–down influences may also underlie the word superiority effect3. Alternatively, it is
possible that lpFg stimulation disrupted naming by interfering with remote processing, and that top–down information to the putative letter-form area serves only as a training signal to help
refine processing that is essentially sensory pattern recognition. In either case, our results suggest that words are processed first sequentially in stages with increasing complexity4, and
then in parallel in multiple areas encoding complementary properties43. METHODS PARTICIPANTS Twelve healthy right-handed subjects underwent fMRI testing, and a separate group of 12 healthy
subjects underwent MEG testing. In addition, we analysed LFP from nine patients implanted with intracranial electrodes while performing the task (Supplementary Table 1 for patient
characteristics). Electrodes were implanted to localize seizure onset before contemplated surgical treatment. One of these patients was also studied with fMRI during the same task before
surgery, and another with both fMRI and MEG. Subjects gave written informed consent to participate in this study, and the study was approved by the New York University Medical Center (NYUMC)
and University of Californa's Instituional Review Boards (UCSD IRBs) in accordance with the Declaration of Helsinki. SEMANTIC JUDGMENT TASK Stimuli were white letters on a black
background in Arial font at 4° visual angle, comprising RW, previously presented ‘old’ words (OW), non-pronounceable consonant letter-strings (CS), FF stimuli and 40 target words. FF were
alphabet-like characters that matched a real letter in the English alphabet in size, number of strokes, total line length and curvature (Table 1). FF strings were each matched to a RW in the
number of characters. Subjects pressed a button in response to low-frequency target words representing animals. RW were 4–8 letter nouns, with a written lexical frequency of 3–80 per 10
million44. Tasks were programmed using Presentation software (Neurobehavioural Systems, Inc). The same design was used for both MEG and iEEG. We presented 400 each RW, OW, CS and FF, plus 80
targets pseudo-randomly with the constraint that each condition was preceded by every other condition with equal likelihood. Stimulus exposure and stimulus onset asynchrony were both 600
ms. Throughout the experiment, each CS and FF stimulus was only presented once. Here we report results on the RW, CS and FF comparisons; later responses to stimulus repetition are reported
elsewhere45. Subjects detected 83% (s.d.=12.2) of the targets in the MEG task with a mean reaction time of 694 ms (s.d.=92 ms). They detected 78% (s.d.=13.8) during iEEG recordings
(chance=4.8%) with a mean reaction time of 744 ms (s.d.=121 ms). As the reaction time (RT) often exceeded the stimulus onsetasynchrony (SOA), the trials following targets were excluded from
averages. A blocked version of the semantic judgment task was designed for fMRI in order to maximize SNR, with 30 blocks including 5 blocks each of RW, OW and CS, and 15 blocks of FF. Each
block contained 40 words of one stimulus type, plus two targets. Blocks of RW and CS were presented in random order. Subjects detected 84% (s.d.=9.2) of the targets. Mean reaction time was
688 ms (s.d.=76 ms). MRI ANALYSIS Twelve healthy subjects (six males, mean age: 23, range 19–36) underwent fMRI testing. Each subject was right-handed and free of neurological impairments.
Handedness was assessed with the Edinburgh Handedness Inventory46. The 3T MRI data were acquired and analysed using FreeSurfer, FSL, and custom software as previously described45.
Letter-specific activation was defined as increased BOLD to CS versus FF, as they were closely matched on basic visual features. Similarly, word-specific activity was defined as increased
BOLD to RW versus CS. Larger responses to FF are common, with EEG, as well as BOLD, especially in the right hemisphere9,15. As such responses are thought to reflect the novelty of FF rather
than template-matching16, we omitted them from our study. Functional MRI data were preprocessed using FSL (www.fmrib.ox.ac.uk/fsl). For each subject, motion correction was performed using
FLIRT47, and data were spatially smoothed using a 5-mm full width half-maximum Gaussian kernel, grand-mean intensity normalized, high-pass filtered at sigma=50 sm and pre-whitened using
FILM48. Functional scans were coregistered to T1-weighted images47,49, and analysed using FMRI Expert Analysis Tool Version 5.90, part of FSLs FMRIB’s software library. BOLD parameter
estimates (beta-weights) were averaged across the two runs for each contrast of interest (RW>CS and CS>FF). Percent signal change was calculated in MATLAB (The Mathworks, Natrick, MA)
by multiplying the beta-weights by 100 × the regressor height and dividing by the mean functional volume. Individually averaged functional data were then resampled from each volume to each
individual’s native surface, then from native surface to spherical atlas space for surface-based group analysis. MEG ANALYSIS MEG signals were recorded from 204 planar gradiometers as
previously described11. Distributed source estimates of cortical activity were calculated from gradiometer data using dynamic statistical parametric mapping and cortical dipole constraints
derived from each individual’s reconstructed MRI17. Peak amplitudes from each subject in fMRI-based regions of interests were entered into analysis of variance. INTRACRANIAL EEG ANALYSIS LFP
were recorded from intracranially implanted subdural electrodes (AdTech medical Instrument Corp., WI, USA) in patients undergoing elective monitoring of medically intractable seizures
(Supplementary Table S1 for patient demographics), with implant sites over the left ventral occipito-temporal cortex in nine patients. A large number of additional brain areas were sampled,
including regions that were subsequently determined to be non-epileptogenic. Patients were native English speaking and left language dominant, with average performance on cognitive, language
and reading tests and normal language organization as indicated by cortical stimulation mapping, when available. Only electrode contacts outside the seizure onset zone and with normal
interictal activity were included in the analysis. In each case, the source of the patient’s epilepsy was thought to be focal and in an operable brain region. Electrode placement was based
entirely on clinical grounds for identification of seizure foci and eloquent cortex during stimulation mapping, and included grid (8 × 8 contacts), depth (1 × 8 contacts) and strip (1 × 4 to
1 × 12 contacts) electrode arrays with 10 mm inter-electrode spacing centre-to-centre. Subdural grid and strip contacts were 4 mm in diameter; consequently the distance between contacts was
6 mm. A large number of brain areas was sampled, with coverage extending widely into regions that were subsequently determined to be non-epileptogenic. All nine patients met the following
strict selection criteria: (1) left language lateralization as indicated by Wada testing; (2) cognitive and language abilities in the average range, including language and reading ability,
as indicated by formal neuropsychological testing (Supplementary Table S1); (3) native English speaking; (4) normal language organization as indicated by cortical stimulation mapping, when
available; (5) above 75% performance on the semantic judgment task; and (6) electrode strips sampling from the left ventral occipito-temporal cortex. In addition, only electrode contacts
outside the seizure onset zone and with normal interictal activity were included in the analysis. EEG activity was recorded at 400 Hz with a Nicolet 128 channel clinical amplifier (0.1
Hz–200 Hz) or at 1000 Hz with a custom-design 256 channel recording system (0.1 Hz–500 Hz). The precise localization of each electrode was computed by coregistering two T1-weighted MRIs, one
obtained preoperatively and one on the day after implant surgery with the electrodes in place. A spatial optimization algorithm was used to integrate known information from the array
geometry and intra-operative photos to achieve high spatial accuracy of the electrode locations in relation to the cortical MRI surface. Electrodes were visualized on the reconstructed pial
surface from T1-weighted MRI scans using Freesurfer v4.1. For anatomical orientation, the Freesurfer generated cortical parcellations were overlaid onto the reconstructed surface (Fig. 4d).
Data were analysed in Matlab using Fieldtrip and custom routines. Statistical comparison across stimulus types used a nonparametric randomization test with temporal clustering. Phase-locking
value23, as well as a single-trial analogue (Supplementary Methods) were calculated between responsive subdural electrode contacts. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Thesen
T. _et al._ Sequential then interactive processing of letters and words in the left fusiform gyrus. _Nat. Commun._ 3:1284 doi: 10.1038/ncomms2220 (2012). REFERENCES * Rosazza C., Appollonio
I., Isella V., Shallice T. Qualitatively different forms of pure alexia. _Cogn. Neuropsychol._ 24, 393–418 (2007). Article Google Scholar * Patterson K., Kay J. Letter-by-letter reading:
psychological descriptions of a neurological syndrome. _Q. J. Exp. Psychol. A._ 34, Part 3411–441 (1982). Article CAS Google Scholar * Grainger J., Jacobs A. M. A dual read-out model of
word context effects in letter perception: further investigations of the word superiority effect. _J. Exp. Psychol.: Hum. Percept. Perform._ 20, 1158–1176 (1994). Google Scholar * Dehaene
S., Cohen L. The unique role of the visual word form area in reading. _Trends Cogn. Sci._ 15, 254–262 (2011). Article Google Scholar * Price C. J., Devlin J. T. The interactive account of
ventral occipitotemporal contributions to reading. _Trends Cogn. Sci._ 15, 246–253 (2011). Article Google Scholar * Binder J. R., Medler D. A, Westbury C. F, Liebenthal E., Buchanan L.
Tuning of the human left fusiform gyrus to sublexical orthographic structure. _Neuroimage_ 33, 739–748 (2006). Article Google Scholar * Glezer L. S., Jiang X., Riesenhuber M. Evidence for
highly selective neuronal tuning to whole words in the ‘visual word form area’. _Neuron_ 62, 199–204 (2009). Article CAS Google Scholar * Vinckier F. et al. Hierarchical coding of letter
strings in the ventral stream: dissecting the inner organization of the visual word-form system. _Neuron_ 55, 143–156 (2007). Article CAS Google Scholar * Bentin S., Mouchetant-Rostaing
Y., Giard M. H., Echallier J. F., Pernier J. ERP manifestations of processing printed words at different psycholinguistic levels: time course and scalp distribution. _J. Cogn. Neurosci._ 11,
235–260 (1999). Article CAS Google Scholar * Tarkiainen A., Helenius P., Hansen P. C., Cornelissen P. L., Salmelin R. Dynamics of letter string perception in the human occipitotemporal
cortex. _Brain_ 122, Part 112119–2132 (1999). Article Google Scholar * Leonard M. K. et al. Spatiotemporal dynamics of bilingual word processing. _Neuroimage_ 49, 3286–3294 (2010). Article
Google Scholar * Allison T., McCarthy G., Nobre A., Puce A., Belger A. Human extrastriate visual cortex and the perception of faces, words, numbers, and colors. _Cereb. Cortex_ 4, 544–554
(1994). Article CAS Google Scholar * Gaillard R. et al. Direct intracranial, FMRI, and lesion evidence for the causal role of left inferotemporal cortex in reading. _Neuron_ 50, 191–204
(2006). Article CAS Google Scholar * Mainy N. et al. Cortical dynamics of word recognition. _Hum. Brain Mapp._ 29, 1215–1230 (2008). Article ADS Google Scholar * Appelbaum L. G.,
Liotti M., Perez R., Fox S. P., Woldorff M. G. The temporal dynamics of implicit processing of non-letter, letter, and word-forms in the human visual cortex. _Front Hum. Neurosci._ 3, 56
(2009). Article Google Scholar * Tagamets M. A., Novick J. M., Chalmers M. L., Friedman R. B. A parametric approach to orthographic processing in the brain: an fMRI study. _J. Cogn.
Neurosci._ 12, 281–297 (2000). Article CAS Google Scholar * Dale A. M. et al. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical
activity. _Neuron_ 26, 55–67 (2000). Article CAS Google Scholar * Dale A. M., Halgren E. Spatiotemporal mapping of brain activity by integration of multiple imaging modalities. _Curr.
Opin. Neurobiol._ 11, 202–208 (2001). Article CAS Google Scholar * Mechelli A., Humphreys G. W., Mayall K., Olson A., Price C. J. Differential effects of word length and visual contrast
in the fusiform and lingual gyri during reading. _Proc. Biol. Sci._ 267, 1909–1913 (2000). Article CAS Google Scholar * Schurz M. et al. A dual-route perspective on brain activation in
response to visual words: evidence for a length by lexicality interaction in the visual word form area (VWFA). _Neuroimage_ 49, 2649–2661 (2010). Article Google Scholar * Bruno J. L.,
Zumberge A., Manis F. R., Lu Z. L., Goldman J. G. Sensitivity to orthographic familiarity in the occipito-temporal region. _Neuroimage_ 39, 1988–2001 (2008). Article Google Scholar *
Mukamel R. et al. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. _Science_ 309, 951–954 (2005). Article CAS ADS Google Scholar * Lachaux J. P.,
Rodriguez E, Martinerie J, Varela F. J. Measuring phase synchrony in brain signals. _Hum. Brain Mapp._ 8, 194–208 (1999). Article CAS Google Scholar * Srinivasan R., Nunez, Silberstein R.
Spatial filtering and neocortical dynamics: estimates of EEG coherence. _IEEE Trans. Biomed. Eng._ 45, 814–826 (1998). Article CAS Google Scholar * Koyama M. S. et al. Reading networks
at rest. _Cereb. Cortex._ 20, 2549–2559 (2010). Article Google Scholar * Vogel A. C., Miezin F. M., Petersen S. E., Schlaggar B. L. The putative visual word form area is functionally
connected to the dorsal attention network. _Cereb Cortex_ 22, 537–549 (2012). Article Google Scholar * Price C. J. et al. Hearing and saying. The functional neuro-anatomy of auditory word
processing. _Brain_ 119, 919–931 (1996). Article Google Scholar * Ben-Shachar M., Dougherty R. F., Deutsch G. K., Wandell B. A. Differential sensitivity to words and shapes in ventral
occipito-temporal cortex. _Cereb Cortex_ 17, 1604–1611 (2007). Article Google Scholar * Brem S. et al. Evidence for developmental changes in the visual word processing network beyond
adolescence. _Neuroimage_ 29, 822–837 (2006). Article Google Scholar * Vartiainen J., Liljeström M., Koskinen M., Renvall H., Salmelin R. Functional magnetic resonance imaging blood
oxygenation level-dependent signal and magnetoencephalography evoked responses yield different neural functionality in reading. _J. Neurosci._ 31, 1048–1058 (2011). Article CAS Google
Scholar * Flowers D. L. et al. Attention to single letters activates left extrastriate cortex. _Neuroimage_ 21, 829–839 (2004). Article CAS Google Scholar * Libertus M. E., Brannon E.
M., Pelphrey K. A. Developmental changes in category-specific brain responses to numbers and letters in a working memory task. _Neuroimage_ 44, 1404–1414 (2009). Article Google Scholar *
van der Mark S. et al. Children with dyslexia lack multiple specializations along the visual word-form (VWF) system. _Neuroimage_ 47, 1940–1949 (2009). Article Google Scholar * Marinkovic
K. et al. Spatiotemporal dynamics of modality-specific and supramodal word processing. _Neuron_ 38, 487–497 (2003). Article CAS Google Scholar * Solomyak O., Marantz A. Evidence for early
morphological decomposition in visual word recognition. _J. Cogn. Neurosci._ 22, 2042–2057 (2010). Article Google Scholar * Sahin N. T., Pinker S., Cash S. S., Schomer D., Halgren E.
Sequential processing of lexical, grammatical, and phonological information within Broca's area. _Science_ 326, 445–449 (2009). Article CAS ADS Google Scholar * Halgren E. et al.
Processing stages underlying word recognition in the anteroventral temporal lobe. _Neuroimage_ 30, 1401–1413 (2006). Article Google Scholar * Chan A. M. et al. First-pass selectivity for
semantic categories in human anteroventral temporal lobe. _J. Neurosci._ 31, 18119–18129 (2011). Article CAS Google Scholar * Grill-Spector K. et al. Differential processing of objects
under various viewing conditions in the human lateral occipital complex. _Neuron_ 24, 187–203 (1999). Article CAS Google Scholar * Klopp J., Marinkovic K., Chauvel P., Nenov V., Halgren
E. Early widespread cortical distribution of coherent fusiform face activity. _Hum. Brain. Mapp._ 11, 286–293 (2000). Article CAS Google Scholar * Halgren E. et al. Spatio-temporal stages
in face and word processing. 1. Depth-recorded potentials in the human occipital, temporal and parietal lobes. _J. Physiol._ 88, 1–50 (1994). CAS Google Scholar * Halgren E. et al.
Spatio-temporal stages in face and word processing. 2. Depth-recorded potentials in the human frontal and Rolandic cortices. _J. Physiol._ 88, 51–80 (1994). CAS Google Scholar * Twomey T.,
Kawabata, D K. J., Price C. J., Devlin J. T. Top–down modulation of ventral occipito-temporal responses during visual word recognition. _Neuroimage_ 55, 1242–1251 (2011). Article Google
Scholar * Francis W. N., Kucera H. _Frequency Analysis Of English Usage: Lexicon and Grammar_ (Houghton Mifflin (1982). * McDonald C. R. et al. Multimodal imaging of repetition priming:
using fMRI, MEG, and intracranial EEG to reveal spatiotemporal profiles of word processing. _Neuroimage_ 53, 707–717 (2010). Article Google Scholar * Oldfield R. C. Ambidexterity in
surgeons. _Lancet_ 1, 655 (1971). Article CAS Google Scholar * Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and
motion correction of brain images. _NeuroImage_ 17, 825–841 (2002). Article Google Scholar * Woolrich M. W., Ripley B. D., Brady M., Smith S. M. Temporal autocorrelation in univariate
linear modeling of FMRI data. _NeuroImage._ 14, 1370–1386 (2001). Article CAS Google Scholar * Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain
images. _Med. Image Anal._ 5, 143–156 (2001). Article CAS Google Scholar * Hauk O., Davis M. H., Ford M., Pulvermüller F., Marslen-Wilson W. D. The time course of visual word recognition
as revealed by linear regression analysis of ERP data. _Neuroimage_ 30, 1383–1400 (2006). Article CAS Google Scholar * Sereno S. C., Rayner K., Posner M. I. Establishing a time-line of
word recognition: evidence from eye movements and event-related potentials. _Neuroreport_ 9, 2195–2200 (1998). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank
Anders Dale and Donald Hagler for analysis tools, and Mark Blumberg and Amy Trongnetrpunya for help with data collection. This research was supported by grants from NIH (NS18741) and FACES.
AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Neurology, Comprehensive Epilepsy Center, New York University, New York, 10016, NY, USA Thomas Thesen, Chad Carlson, Werner Doyle,
William Barr, Orrin Devinsky & Ruben Kuzniecky * Departments of Radiology & Neuroscience, Multimodal Imaging Laboratory, University of California, San Diego, 92037, CA, USA Thomas
Thesen, Carrie R. McDonald, Jason Sherfey, Olga Felsovalyi, Holly Girard & Eric Halgren * Department of Neurology, Massachusetts General Hospital, Harvard Medical School, 02114,
Cambridge, MA, USA Syd Cash * Departments of Radiology and Neuroscience, and Kavli Institute for Mind and Brain, University of California, San Diego, 92037, CA, USA Eric Halgren Authors *
Thomas Thesen View author publications You can also search for this author inPubMed Google Scholar * Carrie R. McDonald View author publications You can also search for this author inPubMed
Google Scholar * Chad Carlson View author publications You can also search for this author inPubMed Google Scholar * Werner Doyle View author publications You can also search for this author
inPubMed Google Scholar * Syd Cash View author publications You can also search for this author inPubMed Google Scholar * Jason Sherfey View author publications You can also search for this
author inPubMed Google Scholar * Olga Felsovalyi View author publications You can also search for this author inPubMed Google Scholar * Holly Girard View author publications You can also
search for this author inPubMed Google Scholar * William Barr View author publications You can also search for this author inPubMed Google Scholar * Orrin Devinsky View author publications
You can also search for this author inPubMed Google Scholar * Ruben Kuzniecky View author publications You can also search for this author inPubMed Google Scholar * Eric Halgren View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Experimental design was done by T.T., C.R.M. and E.H.; data collection by T.T., C.R.M., C.C., W.D.,
S.C., O.F., H.G., W.B., O.D., R.K. and E.H.; data analysis by T.T., C.R.M., C.C., J.S., H.G. and E.H.; and manuscript preparation by T.T., C.R.M., C.C., O.D., E.H. CORRESPONDING AUTHOR
Correspondence to Thomas Thesen. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES, TABLE, METHODS
AND REFERENCES Supplementary Figures S1 and S2, Supplementary Table S1, Supplementary Methods and Supplementary References (PDF 389 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Thesen, T., McDonald, C., Carlson, C. _et al._ Sequential then interactive processing of letters and words in the left fusiform gyrus. _Nat Commun_ 3, 1284
(2012). https://doi.org/10.1038/ncomms2220 Download citation * Received: 11 April 2012 * Accepted: 23 October 2012 * Published: 18 December 2012 * DOI: https://doi.org/10.1038/ncomms2220
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative

:max_bytes(150000):strip_icc():focal(319x0:321x2)/people_social_image-60e0c8af9eb14624a5b55f2c29dbe25b.png)







