- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Foraging, defense and waste disposal are essential for sustaining social insect colonies. Hence, their nest generally has an open structure, wherein specialized castes called workers and
soldiers perform these tasks. However, some social aphids form completely closed galls, wherein hundreds to thousands of insects grow and reproduce for several months in isolation. Why these
social aphids are not drowned by accumulated honeydew has been an enigma. Here we report a sophisticated biological solution to the waste problem in the closed system: the gall inner
surface is specialized for absorbing water, whereby honeydew is promptly removed via the plant vascular system. The water-absorbing closed galls have evolved at least twice independently
among social aphids. The plant-mediated waste removal, which entails insect’s manipulation of plant morphogenesis and physiology, comprises a previously unknown mechanism of nest cleaning,
which can be regarded as ‘extended phenotype’ and ‘indirect social behavior’ of the social aphids.
Many insects form conspicuous galls on their host plants. The galls provide the inducer insects with isolated and exclusive habitat, constant and high-quality food supply, physical barrier
against predators and parasites, and mitigated environmental stresses such as desiccation and temperature fluctuation1. The gall-forming insects manipulate the plant growth and morphogenesis
for their own sake in a sophisticated manner, thereby inducing elaborate plant structures as ‘extended phenotypes’ of the insects2.
Social insects such as bees, wasps, ants and termites generally form elaborate nests, in which some colony members reproduce, whereas other members do not necessarily participate in
reproduction but comprise specialized castes called workers and soldiers. Foraging, defense and waste disposal are essential for sustaining social insect colonies. As the colony members
constantly require a large amount of food from the environment and also produce a large amount of wastes to be disposed outside, the social insect colonies are generally unable to persist in
isolation, and these altruistic individuals actively perform social tasks such as foraging, nursing, housekeeping, waste disposal, colony defense and so on3.
Some aphids also produce altruistic individuals called soldiers, whose primary social role is usually defense, whereas some of them may also perform other social tasks such as waste disposal
and gall repair4,5. Many, if not all, of them form conspicuous galls on their host plants and live socially therein. Aphids feed exclusively on plant phloem sap, and as gall tissue
constantly supplies the inhabitants with plant sap, no foraging outside is needed for the gall-forming social aphids. On the other hand, waste disposal is an essential issue for them,
because the gall inhabitants constantly produce large quantities of honeydew and other wastes. In many gall-forming social aphids, their galls have small openings, through which soldier
nymphs actively dispose honeydew droplets and other colony wastes6,7,8,9,10,11,12.
The enigma here is the presence of social aphids that form completely closed galls. Their galls remain closed for several months until their final dehiscence, and contain hundreds to
thousands of insects. Their soldier nymphs may attack gall-boring predators that occasionally invade their galls, but do not perform gall cleaning13,14,15,16,17,18,19. It is expected that
the large quantity of honeydew excreted by hundreds of aphids would quickly fill up the closed gall cavity. Aphid honeydew usually contains substantial quantities of sugars20, which may
cause contamination and other problems in the aphid galls. Why doesn’t the watery waste drown and kill these social aphids confined in the closed galls?
Here we report a sophisticated biological solution to the waste problem in the closed system: these social aphids have evolved to induce galls with an inner surface specialized for absorbing
water, whereby honeydew waste is promptly removed via the plant vascular system. Our finding unveils a previously unknown mechanism of gall cleaning, which can be regarded as an ‘extended
phenotype’ and a ‘plant-mediated social behavior’ of the social aphids with an imprisoned lifestyle.
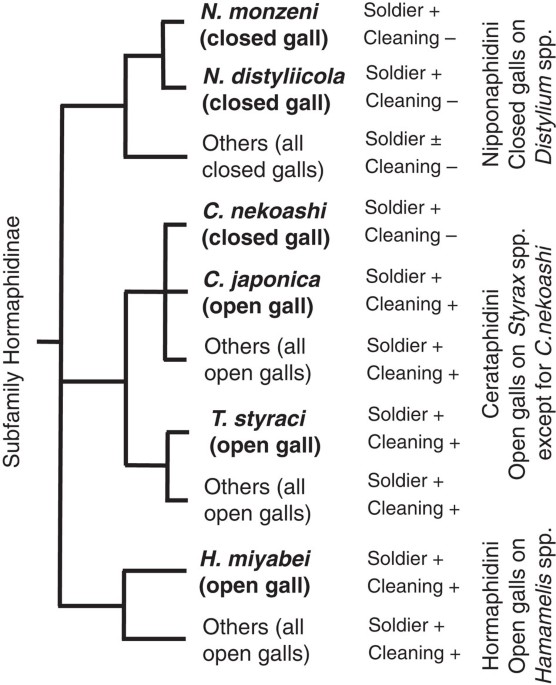
The aphid subfamily Hormaphidinae consists of the tribes Nipponaphidini, Hormaphidini and Cerataphidini. On their primary host plants, these aphids form conspicuous galls that harbor
hundreds, thousands or more colony members: closed galls on Distylium spp. by Nipponaphidini species; open galls on Hamamelis spp. by Hormaphidini species; and open galls on Styrax spp. by
Cerataphidini species (Fig. 1; Supplementary Fig. S1)4. Note that a Cerataphidini species, Ceratovacuna nekoashi, exceptionally forms a banana-shaped closed gall15. Most of the Hormaphidinae
species produce soldier nymphs in their galls, which defend their galls against predators. In the species that form open galls, the soldier nymphs perform an additional social task for
cleaning their galls: pushing wastes such as honeydew droplets, shed skins and dead insects out of the gall through the opening(s)4,5. In the species that form closed galls, by contrast,
such a waste disposal is impossible for several months until the galls open upon maturity.
Species examined in this study representing the tribes Nipponaphidini, Hormaphidini and Cerataphidini of the subfamily Hormaphidinae are shown. Their gall type, soldier production and
gall-cleaning behavior are mapped on the phylogeny. +, − and ± indicate presence of the trait, absence of the trait and some species with the trait while other species without the trait,
respectively. Galls of six species examined in this study, N. monzeni and N. distyliicola from Nipponaphidini, C. nekoashi, C. japonica and T. styraci from Cerataphidini, and H. miyabei from
Hormaphidini are indicated in bold.
Tuberaphis styraci (Cerataphidini) forms large coral-shaped galls on Styrax obassia trees (Fig. 2a). Mature galls often harbor over 10,000 insects, and more than half of them may be second
instar soldier nymphs specialized for colony defense and gall cleaning21,22. On the underside of the galls, there are many openings (Fig. 2b), through which the soldier nymphs dispose
honeydew droplets and other colony wastes7,23. Of 10 galls of T. styraci we examined, all contained a large amount of wax-coated honeydew droplets (Fig. 2c), to which soldier nymphs actively
exhibited cleaning behavior by pushing the droplets with their head (Fig. 2d).
(a) A mature gall of T. styraci formed on S. obassia. (b) Openings located at the projection tips on the underside of a gall of T. styraci. (c) An inside view of a gall of T. styraci. Arrows
indicate honeydew droplets accumulated in the gall. (d) Gall cleaning behavior of a second-instar soldier nymph of T. styraci, pushing a honeydew droplet with its head. (e) A mature gall of
N. monzeni formed on D. racemosum. (f) An inside view of an immature gall of N. monzeni. (g) N. monzeni individuals placed on an artificial diet. Arrows indicate honeydew droplets excreted
by the aphids. (h) An inside view of an immature gall of N. monzeni. Arrows indicate honeydew droplets.
Hamamelistes miyabei (Hormaphidini) forms spiny galls on Hamamelis japonica trees (Fig. 3a). Mature galls often contain over 300 insects, wherein monomorphic first-instar nymphs dispose
honeydew and other wastes through an opening located at the bottom of the gall12. Of eight galls of H. miyabei we inspected, all contained a large mass of wax-coated honeydew (Fig. 3b).
(a) A spiny gall of H. miyabei formed on H. japonica. (b) An inside view of a gall of H. miyabei containing a number of aphids and a large mass of honeydew (arrow). (c) Hydrophobic inner
gall wall of H. miyabei on which added water forms sphere. (d) Transmission electron microscopy of the inner gall wall of H. miyabei, in which a well-defined surface wax layer is seen. (e) A
fig-shaped gall of N. distyliicola formed on D. racemosum. (f) An inside view of a gall of N. distyliicola containing a number of aphids but no honeydew globules. (g) Hydrophilic inner gall
wall of N. distyliicola on which added water spreads and wets the surface. (h) Transmission electron microscopy of the inner gall wall of N. distyliicola, where a spongy wax layer is seen.
CW, cell wall; In, inner side of the gall; PC, plant cell; V, vacuole; WL, waxy surface layer.
These observations indicate that a large number of aphids in the open galls are continuously producing a large amount of honeydew, which must be properly handled and disposed by soldier
nymphs for survival and functioning of the aphid colonies10.
Nipponaphis monzeni (Nipponaphidini) forms closed ball-shaped galls on Distylium racemosum trees (Fig. 2e), wherein monomorphic first-instar nymphs attack gall-boring enemies and perform
self-sacrificing gall repair17,18, but exhibit no gall cleaning behavior. The galls rapidly grow during spring–summer, and the mature galls in autumn often contain over 2,000 insects19.
However, surprisingly, no accumulation of honeydew was observed in both young and mature galls of N. monzeni. Only numerous live insects and powdery aggregates, which consisted mainly of
excreted wax and also of shed skins and dry corpses, were found in the closed galls (Fig. 2f). The possibility that N. monzeni excretes little honeydew in the galls was rejected by the
following observations. When the aphids were placed on an artificial feeding system consisting of a liquid artificial diet sandwiched by Parafilm membranes24, a number of honeydew droplets
soon appeared around the insects (Fig. 2g). Close inspection of young immature galls occasionally identified a small number of honeydew droplets (Fig. 2h).
Nipponaphis distyliicola (Nipponaphidini) forms closed fig-shaped galls on D. racemosum trees (Fig. 3e). Mature galls of N. distyliicola often contain over 700 insects16, wherein monomorphic
first-instar nymphs attack gall-boring enemies13, and as in N. monzeni, no accumulation of honeydew was observed. Only aphids and powdery aggregates were present in the closed galls (Fig.
3f).
These observations suggest that, in the closed galls of the Nipponaphidini aphids, the insects do excrete a considerable amount of honeydew, but the waste is somehow removed from the closed
inner space.
Then, we performed the following field experiments. A small hole was bored on three immature galls of N. monzeni on D. racemosum trees, 1 ml of food dye solution was injected into the galls,
and the hole was immediately filled with an adhesive. When we inspected the galls 5 days later, no water remained inside, and the inner bottom wall of the galls was stained in red.
Subsequent experiments with more galls revealed that the injected water disappeared within 20 h.
To visualize the water absorption routes in detail, we injected 0.5 ml of 0.2% safranin solution into a gall as described above25. After 20 h, no solution remained inside the gall, and,
strikingly, vein-like staining patterns were clearly observed in the gall wall (Fig. 4a). Tissue sectioning of the gall wall unequivocally showed that vascular bundles were selectively
stained with safranin (Fig. 4b).
(a,b) Tracking the route of water absorption in a gall of N. monzeni by safranin staining. (a) An inside view of the gall 20 h after injection of safranin solution inside. Safranin is
precipitated at the bottom of the gall due to water absorption. (b) A histological section of the safranin-injected gall. (c,d) Hydrophobicity of the gall inner surface shown by an addition
of food dye water. (c) A gall of N. monzeni. (d) A gall of T. styraci. (e,f) Transmission electron microscopy of the inner wall of the galls. (e) A gall of N. monzeni with a spongy surface
layer. (f) A gall of T. styraci with a solid surface layer. CW, cell wall; In, inner side of the gall; Out, outer side of the gall; PC, plant cell; WL, waxy surface layer.
These results indicate that the gall wall of N. monzeni is capable of absorbing water from inside through the vascular system, and suggest that the honeydew excreted by gall inhabitants is
removed by the mechanism.
What mechanisms are involved in the water absorption by the gall wall of N. monzeni? A possible mechanism is passive water absorption driven by water potential of the plant tissue, whereas
an alternative mechanism is more specific water absorption mediated by water channels such as aquaporins26.
When water absorption was measured for gall wall preparations in the laboratory, addition of mercury chloride, an aquaporin inhibitor, did not affect the levels of water absorption (Fig. 5).
Moreover, a non-water substance, safranin, was efficiently incorporated into the gall tissue (Fig. 4a). These observations suggest that aquaporin-mediated water transport is unlikely to be
the major mechanism for water absorption by the gall wall of N. monzeni.
Means and s.d. are shown with sample sizes in parentheses. ND means no significant difference (generalized linear model; P>0.05).
Next, 58 immature galls of N. monzeni and 10 immature galls of N. distyliicola were subjected to the following field and laboratory experiments. In the field, 1 ml each of distilled water,
2% (58.5 mM) sucrose water, 4% (117 mM) sucrose water and 8% (234 mM) sucrose water was injected into each of 16, 10, 10 and 11 galls of N. monzeni, respectively. Also in the field, 1 ml of
distilled water was injected into each of 11 galls of N. distyliicola. In addition, 1 ml of distilled water was injected into each of 11 galls of N. monzeni kept on water bottles in the
laboratory. Water remaining in each of the galls was inspected after 20 h. In the distilled water treatment, almost complete absorption (>90%) was observed in 15 of 16 galls of N. monzeni
examined in the field (Fig. 6a), 5 of 11 galls of N. monzeni examined in the laboratory (Fig. 6e) and 4 of 10 galls of N. distyliicola in the field (Fig. 6f). In the 2% sucrose water
treatment, complete absorption occurred in six galls of N. monzeni, whereas the other four galls exhibited 50–90% absorption (Fig. 6b). In the 4% sucrose water treatment, almost complete
absorption was found in two galls, whereas the other eight galls exhibited 35–90% absorption (Fig. 6c). In the 8% sucrose water treatment, all the 11 galls exhibited little water absorption
(Fig. 6d).
(a) Distilled water in galls of N. monzeni, field experiment (n=16). (b) Two percentage (58.5 mM) sucrose water in galls of N. monzeni, field experiment (n=10). (c) Four percentage (117 mM)
sucrose water in galls of N. monzeni, field experiment (n=10). (d) Eight percentage (234 mM) sucrose water in galls of N. monzeni, field experiment (n=11). (e) Distilled water in galls of N.
monzeni, laboratory experiment (n=11). (f) Distilled water in galls of N. distyliicola, field experiment (n=10). The number above each column indicates volume (ml) of the gall.
These results indicate that the effect of water potential is mainly involved in the water absorption by the gall wall of N. monzeni, and probably in that of N. distyliicola. Water potential
quantifies the tendency of water to move from one area to another due to osmosis, gravity, mechanical pressure and matrix effects such as surface tension26. Judging from our data, the water
potential of the gall wall may be roughly equivalent to the osmotic pressure of 8% (234 mM) sucrose water.
Of 2 μl of honeydew sample collected from >30 immature galls of N. monzeni, initially 0.5 μl and next 1.0 μl were analysed by liquid chromatography. In both measurements, no distinct peaks
were detected for fructose, sucrose, maltose, trehalose and melezitose. The only significant peak was that of glucose. The glucose concentration was estimated as 0.12% (6.7 mM) in the first
measurement and as 0.34% (18.9 mM) in the second measurement, respectively (Supplementary Fig. S2). Considering that even 4% (117 mM) sucrose water was efficiently absorbed by the gall wall
(Fig. 6c), it is expected that honeydew of N. monzeni is readily absorbed by and removed from the aphid gall.
As for the open galls of T. styraci, by contrast, no such water absorption was observed. We collected 11 galls of T. styraci, and injected 0.3–1.0 ml of food dye solution depending on the
gall size into each of the galls. To minimize the effects of soldiers’ gall cleaning activity, the dye solution was injected into a gall area that had no gall opening. After 20 h, almost the
same amount of dye solution was recovered from each of the galls. The gall wall of the area exhibited no dye staining. These results indicate no water absorption by the galls of T. styraci.
Notably, when the dye water was applied to the inner gall surface of N. monzeni, the water immediately spread and wet the surface (Fig. 4c). Water droplets placed on the inner gall surface
of N. monzeni exhibited a contact angle of 50.7±16.6° (average±s.d., n=12). By contrast, the dye water was repelled by the inner gall surface of T. styraci, forming water spheres (Fig. 4d).
Water droplets placed on the inner gall surface of T. styraci exhibited a contact angle of 130.8±11.8° (n=13). Transmission electron microscopy revealed a remarkable structural difference in
their gall inner surface: a thick and distinct surface layer, which is presumably made of wax, was observed in the galls of T. styraci (Fig. 4f), whereas the layer was thin, reticular and
spongy in the galls of N. monzeni (Fig. 4e). Interestingly, similar patterns were consistently observed in the galls of other aphid species: hydrophobic inner gall surface exhibiting a water
contact angle of 114.0±10.7° (n=12) with distinct surface layer in the open galls of H. miyabei (Fig. 3c) in contrast to hydrophilic inner gall surface exhibiting a water contact angle of
43.4±13.8° (n=13) with spongy surface layer in the closed galls of N. distyliicola (Fig. 3g).
On the basis of these results, we conclude that in N. monzeni (and possibly also in N. distyliicola), the honeydew excreted by a large number of aphids, which can potentially drown and kill
the aphids in the closed space, is absorbed by plant tissue of the gall inner wall, whereby the gall inside is kept dry and safe. Previous studies on a number of Cerataphidini and
Hormaphidini aphids consistently reported that soldier nymphs have a pivotal role for removing honeydew and other wastes by their gall cleaning behavior7,9,11,12. The plant-mediated honeydew
absorption, which entails insect’s manipulation of plant morphogenesis and physiology, is a previously unknown type of gall cleaning.
In general, Cerataphidini aphids form open galls on their specific Styrax trees5. For example, Ceratovacuna japonica forms banana-bundle-shaped galls on S. japonicus trees (Fig. 7a). Each of
6–12 subgalls has a hollow cavity with a slit opening at the tip (Fig. 7b), and contains from 50 to over 100 aphids inside (Fig. 7c). In the open galls, C. japonica produces second-instar
soldier nymphs, which attack natural enemies as well as perform gall cleaning27. Here it is notable that there is an exceptional Cerataphidini species, C. nekoashi, that is congenic with C.
japonica but forms closed galls on the same S. japonicus trees (Fig. 7f)15. Each of 9–13 subgalls comprises a closed cavity without opening and harbors from 50 to over 100 aphids (Fig. 7g).
The second-instar soldier nymphs of C. nekoashi certainly attack natural enemies, but do not perform gall cleaning14. Molecular phylogenetic analysis confirmed that C. japonica and C.
nekoashi belong to the same monophyletic group consisting of Pseudoregma/Ceratovacuna species in the Cerataphidini (Supplementary Fig. S1). Hence, comparison between C. japonica and C.
nekoashi will provide insights into evolutionary aspects of the open and closed galls.
(a) A banana-shaped gall of C. japonica with 12 subgalls formed on Styrax japonicus. (b) Subgalls of C. japonica with a slit opening on their tip. (c) An inside view of a subgall of C.
japonica containing a number of honeydew droplets and insects. (d) Hydrophobic inner gall wall of C. japonica on which added water forms a sphere. (e) Transmission electron microscopy of the
inner gall wall of C. japonica, in which a well-defined surface wax layer is seen. (f) A banana-shaped gall of C. nekoashi with 11 subgalls formed on S. japonicus. (g) Closed subgalls of C.
nekoashi with no opening. (h) An inside view of a subgall of C. nekoashi, where no honeydew droplets are found despite a number of gall inhabitants. (i) Hydrophilic inner gall wall of C.
nekoashi on which added water spreads and wets the surface. (j) Transmission electron microscopy of the inner gall wall of C. nekoashi, where a spongy surface layer is seen. CW, cell wall;
In, inner side of the gall; PC, plant cell; V, vacuole; WL, waxy surface layer.
In total, we found 69 wax-coated honeydew droplets including 61 small ones (a gall-forming social aphid (T. styraci) >a social aphid that forms water-absorbing closed galls (N. monzeni).
Second, although plant phloem sap is generally sucrose-rich20, the sugar compositions in the honeydew samples were unexpectedly devoid of sucrose, which was particularly conspicuous in
gall-forming social aphids including N. monzeni. Lastly, these gall-forming social aphids generally excrete a plenty of wax in their galls, and thus the low sugar concentrations in the
honeydew of gall-forming social aphids may be attributable to consumption of sucrose for wax production. On the basis of these results and rationales, although speculative, we propose a
hypothesis that the wax production by gall-forming social aphids may contribute to their waste disposal in different ways: in species forming open galls, for making wax-coated honeydew
droplets to facilitate soldier-mediated waste disposal; and in species forming closed galls, for excretion of low-osmotic honeydew to facilitate plant-mediated waste removal. An alternative
hypothesis is that the social aphids forming closed galls may excrete low-osmotic honeydew wherein sugars are polymerized into polysaccharides.
In general, social insect colonies cannot persist for a considerable period in isolation except for the inactive hibernation period, because they constantly require a large amount of food
from the environment and also produce a large amount of wastes to be disposed outside. In the galling social aphids, by contrast, their plant-made nests directly provide the plant sap diet,
the major component of their wastes is aqueous solution, and these peculiar ecological traits must have enabled some of them to evolve a unique strategy of social living in completely closed
galls.
Galls of N. monzeni formed on D. racemosum trees were experimentally manipulated and studied at Shinkiba, Tokyo, Japan, from April to June in 2007 to 2012. After a series of experiments, the
galls were collected, brought to the laboratory at Tsukuba, Ibaraki, Japan, and subjected to further analyses. Galls of other social aphids examined in this study are listed in
Supplementary Table S1.
PCR, cloning and sequencing of a 1.6 kb mitochondrial DNA segment containing small subunit rRNA, tRNA-Val and large subunit rRNA genes were performed as described31. Accession numbers of the
DNA sequences either determined in this study or used for the following phylogenetic analysis are listed in Supplementary Table S2. A multiple alignment of the nucleotide sequences was
generated using the program Clustal W32. Aligned nucleotide sites containing gaps were removed from the data set, and the final alignment was inspected and corrected manually. Phylogenetic
analyses were conducted by maximum likelihood (ML), maximum parsimony and Bayesian (BA) methods using the programs RAxML 7.0.033, PAUP 4.0b1034 and MrBayes 3.1.235, respectively. Bootstrap
tests were performed with 1,000 samplings for the ML and maximum parsimony. Posterior probabilities were estimated for BA. For the ML and BA analyses, the substitution model was chosen on
the basis of the Akaike information criterion with the program jModeltest 0.1.136, which selected the GTR+I+G model.
A small 2 × 2 mm square hole was bored on the wall of N. monzeni galls in the field using a fine edge of a chisel. From the hole, 1 ml of food dye water (0.2% Food Red No. 102, Kyoritsu
Foods), distilled water or sucrose water was injected into the gall cavity using a 5-ml syringe with a 22G needle (Terumo Co.). Then the hole was immediately filled with an adhesive (Wood
Glue, Konishi Co., Ltd; consisting mainly of 41% polyvinyl acetate). For the laboratory experiments, galls of N. monzeni formed on twigs of D. racemosum were brought to the laboratory, and
the twigs were put in water bottles during the experiments. Similarly, galls of T. styraci formed on twigs of S. obassia were collected in the field, and the twigs were kept in water bottles
during the laboratory experiments. In the water absorption experiments, 1 ml of food dye water was injected from an opening into the gall cavity. After 20 h, the injected solutions were
recovered and quantified. In the staining experiment, 0.5 ml of 0.2% safranin solution was injected into a N. monzeni gall as described above. The gall was inspected after 20 h, and frozen
sections (16 μm thick) of the gall tissue were made on a cryostat (HM-505E, Carl Zeiss), mounted on glass slides and observed under a light microscope.
Six galls of N. monzeni were collected on 5 June 2008, brought to the laboratory and subjected to the experiment. Four 1 × 1 cm pieces of the gall wall were cut out of each gall and placed
in a plastic chamber. Onto inner side of the gall pieces, 0.1 ml of distilled water containing either 0 mM, 0.1, 0.3 or 0.5 mM mercury chloride was placed and kept at room temperature.
Simultaneously, 0.1 ml of distilled water containing either 0, 0.1, 0.3 or 0.5 mM mercury chloride was placed on a strip of Parafilm membrane in the same chamber to correct the evaporation
loss of water. Zhang and Tyerman37 reported that 0.1 mM mercury chloride was sufficient to inhibit the water channel activity of aquaporins in plant tissues. Three minutes later, the
solutions were recovered from the gall pieces and the Parafilm membrane, and weighed on a microbalance. The amount of absorbed water was calculated by (water loss on the gall tissue)−(water
loss on the parafilm membrane) in terms of milliliter given a specific gravity of 1. The data were statistically analysed by the software R v. 2.7.238.
Wax-coated honeydew droplets were occasionally found in small immature galls of N. monzeni. From >30 immature galls, 2 μl of honeydew was collected using a fine brush and stored in a plastic
tube at −80 °C until analysis. Honeydew samples from the other aphid species are listed in Supplementary Table S3. The honeydew samples were analysed by high-performance liquid
chromatography essentially as described39. A mixture of six sugar standards (glucose and fructose as monosaccharide, sucrose, maltose and trehalose as disaccharide, and melezitose as
trisaccharide) was used for identification and quantification of sugars in the honeydew samples.
Plant tissue specimens of aphid galls were prefixed by 2.5% glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.2) at 4 °C overnight, and postfixed by 2% osmium tetroxide at 4 °C for 120
min. After dehydration through an ethanol series, the materials were embedded in Spurr resin and cut into ultrathin sections (70 nm thick). The sections were stained with uranyl acetate and
lead citrate, and observed with a transmission electron microscope (H-7000; Hitachi).
Ten microlitre of distilled water was placed on the center of a small piece of inner gall surface, and a digital photograph was taken from a horizontal direction as described30. A contact
angle between the water droplet and the gall wall was measured manually by using a protractor. In general, the surface is regarded as hydrophobic if the contact angle is >90°, and as
hydrophilic if it is





