- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT In nature, complex terpene natural products are formed by the so-called tail-to-head terpene (THT) cyclization. The cationic reaction cascade is promoted efficiently in complex
enzyme pockets, in which cationic intermediates and transition states are stabilized. In solution, the reaction is hard to control and man-made catalysts able to perform selective THT
cyclizations are lacking. We herein report the first example of a successful THT cyclization inside a supramolecular structure. The basic mode of operation in cyclase enzymes was mimicked
successfully and a catalytic non-stop THT was achieved with geranyl acetate as the substrate. The results presented have implications for the postulated reaction mechanism in cyclase
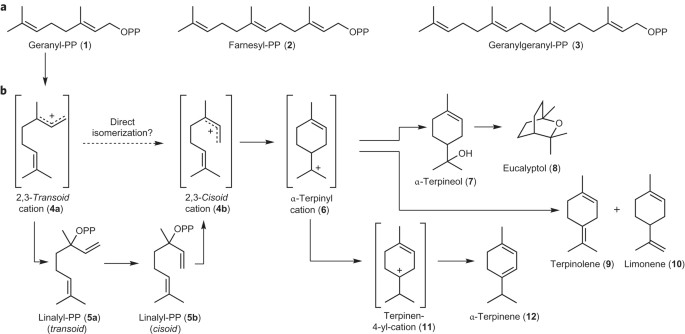
enzymes. Evidence indicates that the direct isomerization of a geranyl cation to the _cisoid_ isomer, which so far was considered unlikely, is feasible. Access through your institution Buy
or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and
online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes
which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY
OTHERS ENZYME-LIKE POLYENE CYCLIZATIONS CATALYZED BY DYNAMIC, SELF-ASSEMBLED, SUPRAMOLECULAR FLUORO ALCOHOL-AMINE CLUSTERS Article Open access 13 February 2023 BIOCATALYTIC STEREOCONTROLLED
HEAD-TO-TAIL CYCLIZATIONS OF UNBIASED TERPENES AS A TOOL IN CHEMOENZYMATIC SYNTHESIS Article Open access 10 June 2024 A MONODOMAIN CLASS II TERPENE CYCLASE ASSEMBLES COMPLEX ISOPRENOID
SCAFFOLDS Article 10 August 2020 REFERENCES * Pronin, S. V. & Shenvi, R. A. Synthesis of highly strained terpenes by non-stop tail-to-head polycyclization. _Nature Chem._ 4, 915–920
(2012). Article CAS Google Scholar * Christianson, D. W. Structural biology and chemistry of the terpenoid cyclases. _Chem. Rev._ 106, 3412–3442 (2006). Article CAS Google Scholar *
Miller, D. J. & Allemann, R. K. Sesquiterpene synthases: passive catalysts or active players? _Nat. Prod. Rep._ 29, 60–71 (2012). Article CAS Google Scholar * Croteau, R. Biosynthesis
and catabolism of monoterpenoids. _Chem. Rev._ 87, 929–954 (1987). Article CAS Google Scholar * Degenhardt, J., Köllner, T. G. & Gershenzon, J. Monoterpene and sesquiterpene
synthases and the origin of terpene skeletal diversity in plants. _Phytochemistry_ 70, 1621–1637 (2009). Article CAS Google Scholar * Cane, D. E. Enzymic formation of sesquiterpenes.
_Chem. Rev._ 90, 1089–1103 (1990). Article CAS Google Scholar * Dickschat, J. S. Isoprenoids in three-dimensional space: the stereochemistry of terpene biosynthesis. _Nat. Prod. Rep._ 28,
1917–1936 (2011). Article CAS Google Scholar * Wise, M. L. & Croteau, R. in _Comprehensive Natural Products Chemistry_ Vol. 2 (eds Barton, D., Nakanishi, K. & Meth-Cohn, O.)
97–153 (Pergamon, 1999). Book Google Scholar * Allinger, N. L. & Siefert, J. H. Organic quantum chemistry. XXXIII. Electronic spectra and rotational barriers of vinylborane, allyl
cation, and related compounds. _J. Am. Chem. Soc._ 97, 752–760 (1975). Article CAS Google Scholar * Lesburg, C. A., Zhai, G., Cane, D. E. & Christianson, D. W. Crystal structure of
pentalenene synthase: mechanistic insights on terpenoid cyclization reactions in biology. _Science_ 277, 1820–1824 (1997). Article CAS Google Scholar * Starks, C. M., Back, K., Chappell,
J. & Noel, J. P. Structural basis for cyclic terpene biosynthesis by tobacco 5-epi-aristolochene synthase. _Science_ 277, 1815–1820 (1997). Article CAS Google Scholar * Hof, F.,
Craig, S. L., Nuckolls, C. & Rebek, J. J. Molecular encapsulation. _Angew. Chem. Int. Ed._ 41, 1488–1508 (2002). Article CAS Google Scholar * Palmer, L. C. & Rebek, J. J. The ins
and outs of molecular encapsulation. _Org. Biomol. Chem._ 2, 3051–3059 (2004). Article CAS Google Scholar * Koblenz, T. S., Wassenaar, J. & Reek, J. N. H. Reactivity within a confined
self-assembled nanospace. _Chem. Soc. Rev._ 37, 247–262 (2008). Article CAS Google Scholar * Rebek, J. Molecular behavior in small spaces. _Acc. Chem. Res._ 42, 1660–1668 (2009). Article
CAS Google Scholar * Yoshizawa, M., Klosterman, J. K. & Fujita, M. Functional molecular flasks: new properties and reactions within discrete, self-assembled hosts. _Angew. Chem. Int.
Ed._ 48, 3418–3438 (2009). Article CAS Google Scholar * Wiester, M. J., Ulmann, P. A. & Mirkin, C. A. Enzyme mimics based upon supramolecular coordination chemistry. _Angew. Chem.
Int. Ed._ 50, 114–137 (2011). Article CAS Google Scholar * Ajami, D. & Rebek, J. More chemistry in small spaces. _Acc. Chem. Res._ 46, 990–999 (2012). Article Google Scholar *
Raynal, M., Ballester, P., Vidal-Ferran, A. & van Leeuwen, P. W. N. M. Supramolecular catalysis. Part 2: artificial enzyme mimics. _Chem. Soc. Rev._ 43, 1734–1787 (2014). Article CAS
Google Scholar * Bocokić, V. et al. Capsule-controlled selectivity of a rhodium hydroformylation catalyst. _Nature Commun._ 4, 2670 (2013). Article Google Scholar * Dydio, P., Detz, R. J.
& Reek, J. N. H. Precise supramolecular control of selectivity in the Rh-catalyzed hydroformylation of terminal and internal alkenes. _J. Am. Chem. Soc._ 135, 10817–10828 (2013).
Article CAS Google Scholar * Wang, Z. J., Clary, K. N., Bergman, R. G., Raymond, K. N. & Toste, F. D. A supramolecular approach to combining enzymatic and transition metal catalysis.
_Nature Chem._ 5, 100–103 (2013). Article CAS Google Scholar * Zhao, C. et al. Chiral amide directed assembly of a diastereo- and enantiopure supramolecular host and its application to
enantioselective catalysis of neutral substrates. _J. Am. Chem. Soc._ 135, 18802–18805 (2013). Article CAS Google Scholar * Zhao, C., Toste, F. D., Raymond, K. N. & Bergman, R. G.
Nucleophilic substitution catalyzed by a supramolecular cavity proceeds with retention of absolute stereochemistry. _J. Am. Chem. Soc._ 136, 14409–14412 (2014). Article CAS Google Scholar
* Salles, A. G., Zarra, S., Turner, R. M. & Nitschke, J. R. A self-organizing chemical assembly line. _J. Am. Chem. Soc._ 135, 19143–19146 (2013). Article CAS Google Scholar *
Hart-Cooper, W. M., Clary, K. N., Toste, F. D., Bergman, R. G. & Raymond, K. N. Selective monoterpene-like cyclization reactions achieved by water exclusion from reactive intermediates
in a supramolecular catalyst. _J. Am. Chem. Soc._ 134, 17873–17876 (2012). Article CAS Google Scholar * MacGillivray, L. R. & Atwood, J. L. A chiral spherical molecular assembly held
together by 60 hydrogen bonds. _Nature_ 389, 469–472 (1997). Article CAS Google Scholar * Cavarzan, A., Scarso, A., Sgarbossa, P., Strukul, G. & Reek, J. N. H. Supramolecular control
on chemo- and regioselectivity via encapsulation of (NHC)-Au catalyst within a hexameric self-assembled host. _J. Am. Chem. Soc._ 133, 2848–2851 (2011). Article CAS Google Scholar *
Bianchini, G., Sorella, G. L., Canever, N., Scarso, A. & Strukul, G. Efficient isonitrile hydration through encapsulation within a hexameric self-assembled capsule and selective
inhibition by a photo-controllable competitive guest. _Chem. Commun._ 49, 5322–5324 (2013). Article CAS Google Scholar * Zhang, Q. & Tiefenbacher, K. Hexameric resorcinarene capsule
is a Brønsted acid: investigation and application to synthesis and catalysis. _J. Am. Chem. Soc._ 135, 16213–16219 (2013). Article CAS Google Scholar * Shivanyuk, A. & Rebek, J.
Reversible encapsulation by self-assembling resorcinarene subunits. _Proc. Natl Acad. Sci. USA_ 98, 7662–7665 (2001). Article CAS Google Scholar * Avram, L. & Cohen, Y. Spontaneous
formation of hexameric resorcinarene capsule in chloroform solution as detected by diffusion NMR. _J. Am. Chem. Soc._ 124, 15148–15149 (2002). Article CAS Google Scholar * Yamanaka, M.,
Shivanyuk, A. & Rebek, J. Kinetics and thermodynamics of hexameric capsule formation. _J. Am. Chem. Soc._ 126, 2939–2943 (2004). Article CAS Google Scholar * Shivanyuk, A. &
Rebek, J. Assembly of resorcinarene capsules in wet solvents. _J. Am. Chem. Soc._ 125, 3432–3433 (2003). Article CAS Google Scholar * Leão Lana, E. J., da Silva Rocha, K. A., Kozhevnikov,
I. V. & Gusevskaya, E. V. Synthesis of 1,8-cineole and 1,4-cineole by isomerization of α-terpineol catalyzed by heteropoly acid. _J. Mol. Catal. A_ 259, 99–102 (2006). Article Google
Scholar * Kelly, B. D., Allen, J. M., Tundel, R. E. & Lambert, T. H. Multicatalytic synthesis of complex tetrahydrofurans involving bismuth(III) triflate catalyzed intramolecular
hydroalkoxylation of unactivated olefins. _Org. Lett._ 11, 1381–1383 (2009). Article CAS Google Scholar * Bugarčić, Z. M., Dunkić, J. D. & Mojsilović, B. M. A simple, convenient and
expeditious approach to cineol. _Heteroatom Chem._ 15, 468–470 (2004). Article Google Scholar * Eschenmoser, A., Ruzicka, L., Jeger, O. & Arigoni, D. Zur Kenntnis der Triterpene. 190.
Mitteilung. Eine stereochemische Interpretation der biogenetischen Isoprenregel bei den Triterpenen. _Helv. Chim. Acta_ 38, 1890–1904 (1955). Article CAS Google Scholar * Croteau, R.
Evidence for the ionization steps in monoterpene cyclization reactions using 2-fluorogeranyl and 2-fluorolinalyl pyrophosphates as substrates. _Arch. Biochem. Biophys._ 251, 777–782 (1986).
Article CAS Google Scholar * Poulter, C. D. & King, C. H. R. Model studies of terpene biosynthesis. A stepwise mechanism for cyclization of nerol to α-terpineol. _J. Am. Chem. Soc._
104, 1422–1424 (1982). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This project was supported by the Bayerischen Akademie der Wissenschaften (Junges Kolleg), Fonds der
Chemischen Industrie (Sachkostenzuschuss), the TUM Junior Fellow Fund and the Dr.-Ing. Leonhard-Lorenz-Stiftung. The help of J. Richers with the graphical design is gratefully acknowledged.
AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department Chemie, Technische Universität München, Lichtenbergstraße 4, Garching, D-85747, Germany Q. Zhang & K. Tiefenbacher Authors * Q.
Zhang View author publications You can also search for this author inPubMed Google Scholar * K. Tiefenbacher View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS K.T. conceived the project and wrote the manuscript with Q.Z. Q.Z. planned and carried out the experiments. K.T. and Q.Z. discussed the experiments and results.
CORRESPONDING AUTHOR Correspondence to K. Tiefenbacher. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY
INFORMATION Supplementary information (PDF 5155 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhang, Q., Tiefenbacher, K. Terpene cyclization
catalysed inside a self-assembled cavity. _Nature Chem_ 7, 197–202 (2015). https://doi.org/10.1038/nchem.2181 Download citation * Received: 15 September 2014 * Accepted: 12 January 2015 *
Published: 16 February 2015 * Issue Date: March 2015 * DOI: https://doi.org/10.1038/nchem.2181 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content:
Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative







