- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
The great promise of current gene transfer methods for the development of truly effective human gene therapy has been hampered by many factors, foremost among which has been the generally
inefficient and nonspecific delivery of gene delivery vectors in vivo. Whereas an ex vivo model for retrovirus-mediated gene delivery has recently indicated very encouraging progress toward
a definitive genetic treatment of children with an immunological disorder1 and direct in vivo delivery of clotting Factor IX with an adeno-associated virus vector has also shown great
promise2, the broad application of genetic transfer methods to the correction of human disease will require the design of vectors that can survive the rigors of production and the onslaughts
of host defenses and that can make their way specifically and efficiently to the intended cells in the body to deliver their therapeutic genes. Virologists have begun to develop methods to
modify viral coat proteins to improve the gene delivery properties of virus vectors3,4,5, but all too often there is insufficient understanding of virus structure and function to produce
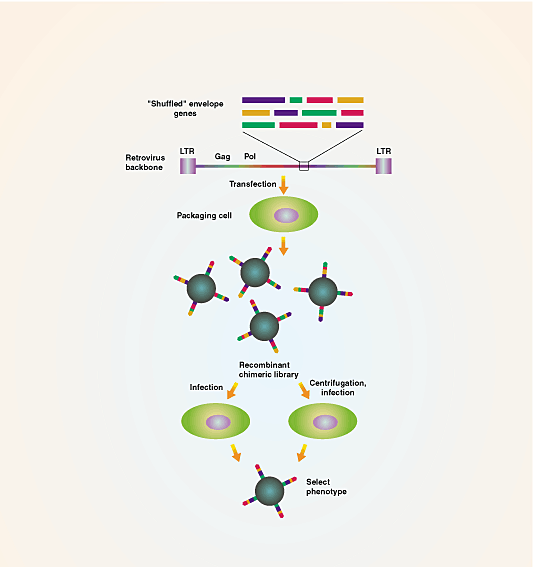
stable, highly concentrated preparations of tissue-targeted vectors. The paper by Powell et al.6 in this issue presents a potentially powerful approach for solving some of the inadequacies
of current protocols letting nature do the work of designing virus envelope proteins that improve the properties of vectors through a process of in vitro genetic recombination called DNA
shuffling.
It has proved generally difficult, but not impossible, to produce retrovirus vectors at the high titers necessary for effective in vivo gene delivery (109–1010 infectious units per ml or
more). A reason for this inadequacy is the presumed structural instability of retroviral envelope proteins that recognize and interact with receptors on the cell surface and that thereby
allow virus attachment and cell uptake. In the well-studied mouse type C Moloney murine leukemia virus (MLV) retrovirus system, the parent for most current retrovirus gene transfer vectors,
loss of virus during concentration has been thought to result from dissociation of the surface (SU) and transmembrane (TM) components of the envelope protein that are ordinarily held
together by a labile disulfide bond. One extremely useful solution to this problem for retrovirus and lentivirus vectors has been the substitution of the retroviral envelope gene with a
“tougher” surrogate envelope protein, the G protein of vesicular stomatitis virus (VSV-G), to produce “pseudotyped” vectors7. Partly because VSV-G consists of a single polypeptide chain
rather than two disulphide-linked chains, it is stable under the shearing forces used to concentrate virus by ultracentrifugation and therefore allows preparation of vector to titers greater
than 109 infectious units per ml. Since the still unidentified cell surface receptor for VSV-G is found on most eukaryotic cells, mammalian and otherwise, VSV-G pseudotyped vectors are
highly promiscuous in their cell tropism.
Anyone you share the following link with will be able to read this content:





