- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Rapid eye movement (REM) sleep is a distinct brain state characterized by activated electroencephalogram and complete skeletal muscle paralysis, and is associated with vivid
dreams1,2,3. Transection studies by Jouvet first demonstrated that the brainstem is both necessary and sufficient for REM sleep generation2, and the neural circuits in the pons have since
been studied extensively4,5,6,7,8. The medulla also contains neurons that are active during REM sleep9,10,11,12,13, but whether they play a causal role in REM sleep generation remains
unclear. Here we show that a GABAergic (γ-aminobutyric-acid-releasing) pathway originating from the ventral medulla powerfully promotes REM sleep in mice. Optogenetic activation of ventral
medulla GABAergic neurons rapidly and reliably initiated REM sleep episodes and prolonged their durations, whereas inactivating these neurons had the opposite effects. Optrode recordings
from channelrhodopsin-2-tagged ventral medulla GABAergic neurons showed that they were most active during REM sleep (REMmax), and during wakefulness they were preferentially active during
eating and grooming. Furthermore, dual retrograde tracing showed that the rostral projections to the pons and midbrain and caudal projections to the spinal cord originate from separate
ventral medulla neuron populations. Activating the rostral GABAergic projections was sufficient for both the induction and maintenance of REM sleep, which are probably mediated in part by
inhibition of REM-suppressing GABAergic neurons in the ventrolateral periaqueductal grey. These results identify a key component of the pontomedullary network controlling REM sleep. The
capability to induce REM sleep on command may offer a powerful tool for investigating its functions. Access through your institution Buy or subscribe This is a preview of subscription
content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 51 print issues and online access $199.00 per year only $3.90 per issue
Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL
ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS A MEDULLARY HUB FOR CONTROLLING REM SLEEP
AND PONTINE WAVES Article Open access 03 July 2023 GABAERGIC NEURONS IN THE ROSTROMEDIAL TEGMENTAL NUCLEUS ARE ESSENTIAL FOR RAPID EYE MOVEMENT SLEEP SUPPRESSION Article Open access 07
December 2022 A CLUSTER OF MESOPONTINE GABAERGIC NEURONS SUPPRESSES REM SLEEP AND CURBS CATAPLEXY Article Open access 25 October 2022 REFERENCES * Aserinsky, E. & Kleitman, N. Regularly
occurring periods of eye motility, and concomitant phenomena, during sleep. _Science_ 118, 273–274 (1953) Article CAS ADS Google Scholar * Jouvet, M. Recherches sur les structures
nerveuses et les mécanismes responsables des différentes phases du sommeil physiologique. _Arch. Ital. Biol._ 100, 125–206 (1962) CAS PubMed Google Scholar * Dement, W. The occurrence of
low voltage, fast, electroencephalogram patterns during behavioral sleep in the cat. _Electroencephalogr. Clin. Neurophysiol._ 10, 291–296 (1958) Article CAS Google Scholar * Hobson, J.
A., McCarley, R. W. & Wyzinski, P. W. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. _Science_ 189, 55–58 (1975) Article CAS ADS Google Scholar *
Boissard, R., Fort, P., Gervasoni, D., Barbagli, B. & Luppi, P. H. Localization of the GABAergic and non-GABAergic neurons projecting to the sublaterodorsal nucleus and potentially
gating paradoxical sleep onset. _Eur. J. Neurosci._ 18, 1627–1639 (2003) Article Google Scholar * Clément, O., Sapin, E., Bérod, A., Fort, P. & Luppi, P. H. Evidence that neurons of
the sublaterodorsal tegmental nucleus triggering paradoxical (REM) sleep are glutamatergic. _Sleep_ 34, 419–423 (2011) Article Google Scholar * Lu, J., Sherman, D., Devor, M. & Saper,
C. B. A putative flip-flop switch for control of REM sleep. _Nature_ 441, 589–594 (2006) Article CAS ADS Google Scholar * Van Dort, C. J. et al. Optogenetic activation of cholinergic
neurons in the PPT or LDT induces REM sleep. _Proc. Natl Acad. Sci. USA_ 112, 584–589 (2015) Article CAS ADS Google Scholar * Siegel, J. M., Wheeler, R. L. & McGinty, D. J. Activity
of medullary reticular formation neurons in the unrestrained cat during waking and sleep. _Brain Res._ 179, 49–60 (1979) Article CAS Google Scholar * Sakai, K., Kanamori, N. & Jouvet,
M. Activités unitaires spécifiques du sommeil paradoxal dans la formation réticulée bulbaire chez le chat non-restreint. _C.R. Seances Acad. Sci. D_ 289, 557–561 (1979) CAS PubMed Google
Scholar * Maloney, K. J., Mainville, L. & Jones, B. E. c-Fos expression in GABAergic, serotonergic, and other neurons of the pontomedullary reticular formation and raphe after
paradoxical sleep deprivation and recovery. _J. Neurosci._ 20, 4669–4679 (2000) Article CAS Google Scholar * Sapin, E. et al. Localization of the brainstem GABAergic neurons controlling
paradoxical (REM) sleep. _PLoS One_ 4, e4272 (2009) Article ADS Google Scholar * Sirieix, C., Gervasoni, D., Luppi, P. H. & Léger, L. Role of the lateral paragigantocellular nucleus
in the network of paradoxical (REM) sleep: an electrophysiological and anatomical study in the rat. _PLoS One_ 7, e28724 (2012) Article CAS ADS Google Scholar * Jego, S. et al.
Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. _Nature Neurosci._ 16, 1637–1643 (2013) Article CAS Google Scholar * Armbruster, B. N.,
Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. _Proc. Natl
Acad. Sci. USA_ 104, 5163–5168 (2007) Article ADS Google Scholar * Kovács, K. J. c-Fos as a transcription factor: a stressful (re)view from a functional map. _Neurochem. Int._ 33, 287–297
(1998) Article Google Scholar * Holmes, C. J. & Jones, B. E. Importance of cholinergic, GABAergic, serotonergic and other neurons in the medial medullary reticular formation for
sleep-wake states studied by cytotoxic lesions in the cat. _Neuroscience_ 62, 1179–1200 (1994) Article CAS Google Scholar * Aston-Jones, G., Ennis, M., Pieribone, V. A., Nickell, W. T.
& Shipley, M. T. The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network. _Science_ 234, 734–737 (1986) Article CAS ADS Google Scholar *
Aston-Jones, G. & Bloom, F. E. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. _J. Neurosci._ 1,
876–886 (1981) Article CAS Google Scholar * Loewy, A. D., Wallach, J. H. & McKellar, S. Efferent connections of the ventral medulla oblongata in the rat. _Brain Res. Rev._ 3, 63–80
(1981) Article Google Scholar * Magoun, H. W. & Rhines, R. An inhibitory mechanism in the bulbar reticular formation. _J. Neurophysiol._ 9, 165–171 (1946) Article CAS Google Scholar
* Schenkel, E. & Siegel, J. M. REM sleep without atonia after lesions of the medial medulla. _Neurosci. Lett._ 98, 159–165 (1989) Article CAS ADS Google Scholar * Vanni-Mercier,
G., Sakai, K., Lin, J. S. & Jouvet, M. Carbachol microinjections in the mediodorsal pontine tegmentum are unable to induce paradoxical sleep after caudal pontine and prebulbar
transections in the cat. _Neurosci. Lett._ 130, 41–45 (1991) Article CAS Google Scholar * Webster, H. H., Friedman, L. & Jones, B. E. Modification of paradoxical sleep following
transections of the reticular formation at the pontomedullary junction. _Sleep_ 9, 1–23 (1986) Article CAS Google Scholar * Kohyama, J., Lai, Y. Y. & Siegel, J. M. Inactivation of the
pons blocks medullary-induced muscle tone suppression in the decerebrate cat. _Sleep_ 21, 695–699 (1998) Article CAS Google Scholar * Siegel, J. M., Nienhuis, R. & Tomaszewski, K. S.
Rostral brainstem contributes to medullary inhibition of muscle tone. _Brain Res._ 268, 344–348 (1983) Article CAS Google Scholar * Carter, M. E. et al. Tuning arousal with optogenetic
modulation of locus coeruleus neurons. _Nature Neurosci._ 13, 1526–1533 (2010) Article CAS Google Scholar * White, S. R., Fung, S. J. & Barnes, C. D. Norepinephrine effects on spinal
motoneurons. _Prog. Brain Res._ 88, 343–350 (1991) Article CAS Google Scholar * Kaur, S., Saxena, R. N. & Mallick, B. N. GABAergic neurons in prepositus hypoglossi regulate REM sleep
by its action on locus coeruleus in freely moving rats. _Synapse_ 42, 141–150 (2001) Article CAS Google Scholar * Taheri, S., Zeitzer, J. M. & Mignot, E. The role of hypocretins
(orexins) in sleep regulation and narcolepsy. _Annu. Rev. Neurosci._ 25, 283–313 (2002) Article CAS Google Scholar * Franklin, K.B.J. & Paxinos, G. _The Mouse Brain in Stereotaxic
Coordinates 3rd_ edn, 88 (Academic Press, 2007) * Miyamichi, K. et al. Dissecting local circuits: parvalbumin interneurons underlie broad feedback control of olfactory bulb output. _Neuron_
80, 1232–1245 (2013) Article CAS Google Scholar * Anikeeva, P. et al. Optetrode: a multichannel readout for optogenetic control in freely moving mice. _Nature Neurosci._ 15, 163–170
(2012) Article CAS Google Scholar * Schmitzer-Torbert, N., Jackson, J., Henze, D., Harris, K. & Redish, A. D. Quantitative measures of cluster quality for use in extracellular
recordings. _Neuroscience_ 131, 1–11 (2005) Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank A. Popescu for the help with _in vivo_ physiology, M. Bikov and S.
Chung for technical assistance, the University of North Carolina Virus Core for supplying AAV, and T. Kilduff and J. Cox for discussions. This work was supported by EMBO and Human Frontier
Science Program postdoctoral fellowships (to F.W.). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Division of Neurobiology, Department of Molecular and Cell Biology, Helen Wills Neuroscience
Institute, Howard Hughes Medical Institute, University of California, Berkeley, 94720, California, USA Franz Weber, Shinjae Chung, Min Xu & Yang Dan * Department of Biology, Howard
Hughes Medical Institute, Stanford University, Stanford, 94305, California, USA Kevin T. Beier & Liqun Luo Authors * Franz Weber View author publications You can also search for this
author inPubMed Google Scholar * Shinjae Chung View author publications You can also search for this author inPubMed Google Scholar * Kevin T. Beier View author publications You can also
search for this author inPubMed Google Scholar * Min Xu View author publications You can also search for this author inPubMed Google Scholar * Liqun Luo View author publications You can also
search for this author inPubMed Google Scholar * Yang Dan View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS F.W. and Y.D. conceived and
designed the experiments. F.W. performed all optogenetic stimulation experiments and optrode recordings. S.C. performed a subset of pharmacogenetic experiments and fluorescence microscopy.
K.T.B. and L.L. provided viral reagents for rabies-mediated trans-synaptic experiments. M.X. designed the optrodes used in this study. F.W. and Y.D. wrote the manuscript, and all authors
participated in the revision of the manuscript. CORRESPONDING AUTHOR Correspondence to Yang Dan. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests.
ADDITIONAL INFORMATION All primary histological, electrophysiological, and behavioural data have been archived in the Department of Molecular and Cell Biology, University of California,
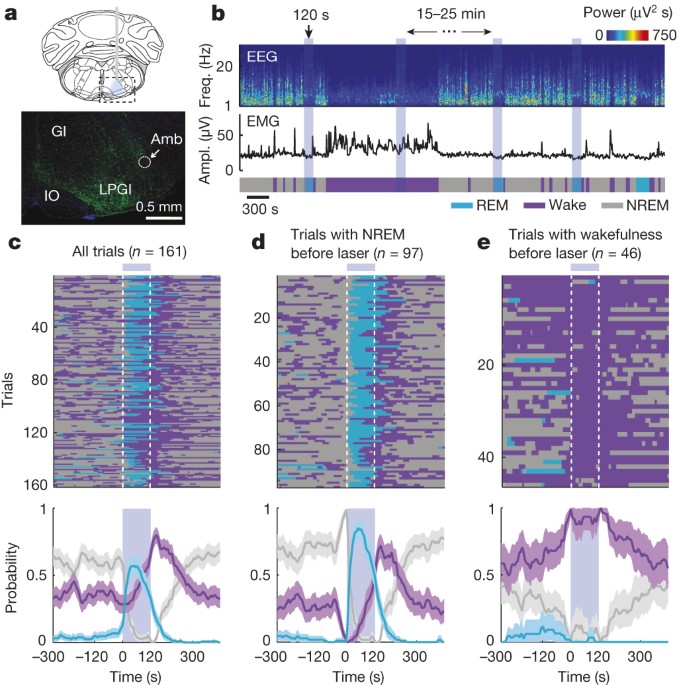
Berkeley. EXTENDED DATA FIGURES AND TABLES EXTENDED DATA FIGURE 1 COMPARISON OF SPONTANEOUS AND LASER-INDUCED REM SLEEP. A, Mean EEG spectrogram and EMG amplitude before and after laser
onset (averaged across all trials with laser onset falling on NREM sleep). B, Mean EEG spectrogram and EMG amplitude before and after spontaneous REM onset outside laser stimulation periods
(only REM episodes with duration >70 s were included). C, Comparison of EEG power spectra during spontaneous (grey) and laser-induced (blue) REM sleep. Blue shading, s.e.m. for
laser-induced REM sleep. EXTENDED DATA FIGURE 2 EFFECT OF LASER STIMULATION ON BRAIN STATES IN EYFP CONTROL MICE. A, Brain states in all trials from five mice aligned by time of laser
stimulation (top) and probability of wake, NREM, or REM states before, during, and after laser stimulation (bottom). Shading, 95% CI. Blue bar, period of laser stimulation. Laser stimulation
caused no significant change in the probability of any brain state (_P_ > 0.34, bootstrap). B, Similar to A, for trials in which laser onset fell on NREM sleep. C, Trials in which laser
onset fell on wakefulness. EXTENDED DATA FIGURE 3 OPTOGENETIC ACTIVATION OF VM GLUTAMATERGIC NEURONS INDUCES WAKEFULNESS. A, Probability of wake, NREM, or REM states before, during, and
after laser stimulation (20 Hz, 120 s) in VGLUT2-Cre mice injected with AAV expressing ChR2–eYFP into the vM (_n_ = 3 mice). Shading, 95% CI. Blue bar, period of laser stimulation. Laser
stimulation caused a significant increase in wakefulness (_P_ < 0.001, bootstrap) and decrease in NREM sleep (_P_ < 0.001). B, Mean durations of REM sleep episodes with and without
laser stimulation. Each pair of dots represents data from one mouse. Laser stimulation shortened the duration of REM sleep episodes (_n_ = 3 mice, _P_ = 0.008, paired _t_-test). Error bar,
s.d. C, Probability of wake (left), NREM (middle), and REM (right) states before, during, and after laser stimulation (20 Hz, 120 s) at different laser powers (colour coded). EXTENDED DATA
FIGURE 4 EFFECT OF LASER STIMULATION OF VM GABAERGIC NEURONS ON THE TRANSITION PROBABILITY BETWEEN EACH PAIR OF BRAIN STATES IN CHR2 AND EYFP CONTROL MICE. A–F, ChR2 control mice. A,
Probability of NREM (N) to REM (R) state transition within each 20 s period in ChR2 mice. Blue shading, period of laser stimulation (20 Hz, 120 s). Error bar, s.d. (bootstrap, _n_ = 6 mice).
Probability of baseline transition (red dashed line) was computed after excluding the laser stimulation period. The probability during laser stimulation was significantly higher than the
baseline (_P_ = 0.02, bootstrap). B, Similar to A, for NREM to wake (W) transition. The probability during laser stimulation was not significantly different from baseline (_P_ = 0.24). C,
REM to wake, probability during laser stimulation was significantly lower than baseline (_P_ < 0.001), consistent with the effect of vM GABAergic neurons on prolonging REM duration (Fig.
2). D, REM to NREM, which rarely occurs in rodents. Laser stimulation caused no significant effect (_P_ > 0.99). E, Wake to REM, which rarely occurs in normal mice. Laser stimulation had
no significant effect (_P_ > 0.99). F, Wake to NREM. Laser stimulation caused a significant reduction in the transition probability (_P_ < 0.001), indicating that during wakefulness vM
GABAergic neuron activity has a wake-maintenance effect. G–L, Similar to A–F, for eYFP control mice. Laser stimulation had no significant effect on any transition probability (_P_ >
0.05). EXTENDED DATA FIGURE 5 PHARMACOGENETIC INACTIVATION OF VM GABAERGIC NEURONS REDUCES REM SLEEP. A, Brain states in a control (vehicle injection) and a CNO session from an example
mouse. The recording session started 20 min after vehicle or CNO injection. B, Probability of each brain state during the first 2.5 h of the recording session, after injection of vehicle
(grey) or two dosages of CNO (different shades of blue). Error bar, s.e.m. (_n_ = 6 mice). *_P_ < 0.05; **_P_ < 0.01; one-way analysis of variance with post hoc Dunnett’s test. C,
Similar to B, but during the second half of the recording session (2.5–5 h). There was no significant difference between control and CNO at any dosage (_P_ > 0.12). D, Latency of first
REM sleep episode (from the beginning of each recording session). E, Frequency of REM episodes during the first 2.5 h of the recording session. F, Duration of REM episodes during the first
2.5 h of the session. The reduction of REM sleep caused by pharmacogenetic inactivation of vM GABAergic neurons appears to be due to the reduction of frequency rather than duration of REM
episodes. EXTENDED DATA FIGURE 6 OPTOGENETIC IDENTIFICATION OF VM GABAERGIC NEURONS. A, Left, reliability and temporal jitter of laser-evoked spikes in identified (blue) and unidentified
(grey) units recorded in the vM. Note that the identified and unidentified units form two distinct clusters, with high reliability and low jitter for identified units. Dark/light symbols,
data during 30/15 Hz laser stimulation. Middle, distribution of delays of laser-evoked spiking for 21 identified GABAergic neurons. Delay is defined as timing of the first spike after each
laser pulse. Right, distribution of correlation coefficient between laser-evoked and spontaneous spike waveforms for all 21 identified vM GABAergic neurons. B, Fluorescence image of a
coronal section showing the position of an electrolytic lesion at the end of the optrode tract (arrow). Blue, DAPI staining. C, Positions of the 21 identified vM GABAergic neurons from 5
mice. Each dot indicates one neuron. All 20 neurons with recording periods encompassing all three brain states showed maximal firing rates during REM sleep. The wakeful behaviour during
which the neuron showed the maximal firing rate is colour-coded. Black, neurons for which the recording period did not include all wakeful behaviours. Schemes of brain sections adapted from
Allen Mouse Brain Atlas (Website: © 2015 Allen Institute for Brain Science. Allen Mouse Brain Atlas [Internet]. Available from: http://mouse.brain-map.org). EXTENDED DATA FIGURE 7 ACTIVITY
OF VM GABAERGIC NEURONS AT REM SLEEP ONSET AND OFFSET. A, Mean firing rates of vM GABAergic neurons at REM onset. Shading, s.e.m. Dark blue, period in which firing rate was significantly
higher than baseline (_P_ < 0.05, Wilcoxon signed-rank test); baseline was defined as the average firing rate during the 10 s intervals 60 s before and after REM sleep. B, Mean firing
rates at REM offset. EXTENDED DATA FIGURE 8 EFFECT OF LASER STIMULATION OF VM NEURONS ON SEVERAL WAKEFUL BEHAVIOURS. A, Probability of moving, running, eating, and grooming before, during,
and after laser stimulation (20 Hz, 120 s) in GAD2-Cre mice injected with AAV expressing ChR2–eYFP into the vM (_n_ = 8 mice). Unclassified behaviours are not shown. Blue bar, period of
laser stimulation. Laser stimulation caused a significant increase in eating (_P_ = 0.008, Wilcoxon signed-rank test). B, Similar to A, but in control mice expressing eYFP (_n_ = 4 mice).
EXTENDED DATA FIGURE 9 FIRING RATES OF UNIDENTIFIED VM NEURONS. A, Firing rates of unidentified units in the three brain states. Each line represents data from one neuron. Gray bar
represents average over units (_n_ = 24). B, Left, relative firing rates of the units across brain states. The firing rates of each unit were normalized by its maximum. Right, normalized
firing rates across different wakeful behaviours. C, Mean firing rates of unidentified vM neurons at REM onset. Shading, s.e.m. Dark blue, period in which firing rate was significantly
higher than baseline (_P_ < 0.05, Wilcoxon signed-rank test). D, Mean firing rates of unidentified vM neurons at REM offset. EXTENDED DATA FIGURE 10 CO-EXPRESSION OF GABA AND GLYCINE IN
VM NEURONS. A, Fluorescence image of ChR2–eYFP expressing neurons in the vM of a GAD2-Cre mouse injected with Cre-inducible AAV. B, Immunohistochemical staining for glycine. C, Superposition
of eYFP expression and glycine staining. In total, 94% (273/289) of eYFP-expressing cells were glycine positive (_n_ = 3 mice). SUPPLEMENTARY INFORMATION OPTOGENETIC ACTIVATION OF VM
GABAERGIC NEURONS INDUCES REM SLEEP The video shows 3 laser stimulation trials, including 1 min before and after each laser stimulation period. In the first two trials the animal was in NREM
sleep at laser onset, and he was awake in the third trial. The EEG spectrogram, EMG amplitude and hypnogram are shown on the right. Laser stimulation periods are depicted by the blue
shading on the top right and additionally indicated as a blue square in the upper right corner of the movie frame; 10x speedup. (MOV 2003 kb) FIRING RATES OF AN IDENTIFIED VM GABAERGIC
NEURON DURING SLEEP AND WAKEFUL BEHAVIORS The video shows two recording periods of an identified unit. During the first period the animal was asleep, during the second period he was awake
and engaged in multiple behaviors (ET – eating, GR – grooming, MV – moving, RU – running, QA – quiet awake). EEG spectrogram, EMG amplitude, hypnogram and firing rate are shown on the right.
The time points of single spikes are represented as vertical lines on the bottom left. Laser stimulation periods (15 or 30 Hz) are shown as blue shadings along with the firing rate and are
indicated by a blue square within the movie frame; 10x speedup. (MOV 2400 kb) POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT
SLIDE FOR FIG. 4 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Weber, F., Chung, S., Beier, K. _et al._ Control of REM sleep by ventral medulla
GABAergic neurons. _Nature_ 526, 435–438 (2015). https://doi.org/10.1038/nature14979 Download citation * Received: 14 May 2015 * Accepted: 23 June 2015 * Published: 07 October 2015 * Issue
Date: 15 October 2015 * DOI: https://doi.org/10.1038/nature14979 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative