- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
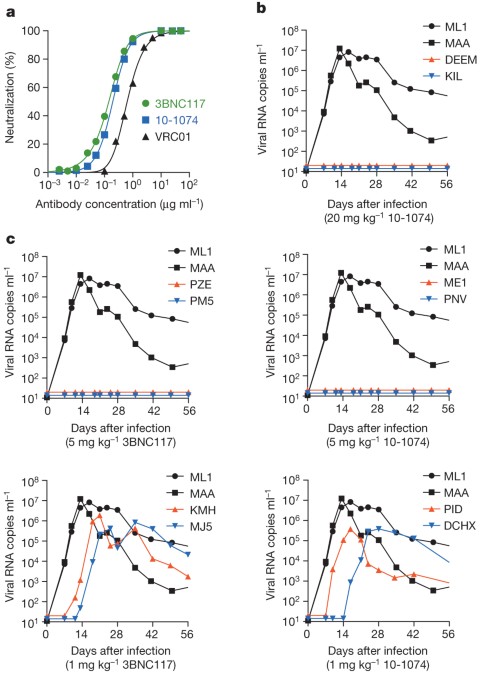
ABSTRACT Neutralizing antibodies can confer immunity to primate lentiviruses by blocking infection in macaque models of AIDS1,2,3,4. However, earlier studies of anti-human immunodeficiency
virus type 1 (HIV-1) neutralizing antibodies administered to infected individuals or humanized mice reported poor control of virus replication and the rapid emergence of resistant
variants5,6,7. A new generation of anti-HIV-1 monoclonal antibodies, possessing extraordinary potency and breadth of neutralizing activity, has recently been isolated from infected
individuals8. These neutralizing antibodies target different regions of the HIV-1 envelope glycoprotein including the CD4-binding site, glycans located in the V1/V2, V3 and V4 regions, and
the membrane proximal external region of gp41 (refs 9, 10, 11, 12, 13, 14). Here we have examined two of the new antibodies, directed to the CD4-binding site and the V3 region (3BNC117 and
10-1074, respectively), for their ability to block infection and suppress viraemia in macaques infected with the R5 tropic simian–human immunodeficiency virus (SHIV)-AD8, which emulates many
of the pathogenic and immunogenic properties of HIV-1 during infections of rhesus macaques15,16. Either antibody alone can potently block virus acquisition. When administered individually
to recently infected macaques, the 10-1074 antibody caused a rapid decline in virus load to undetectable levels for 4–7 days, followed by virus rebound during which neutralization-resistant
variants became detectable. When administered together, a single treatment rapidly suppressed plasma viraemia for 3–5 weeks in some long-term chronically SHIV-infected animals with low CD4+
T-cell levels. A second cycle of anti-HIV-1 monoclonal antibody therapy, administered to two previously treated animals, successfully controlled virus rebound. These results indicate that
immunotherapy or a combination of immunotherapy plus conventional antiretroviral drugs might be useful as a treatment for chronically HIV-1-infected individuals experiencing immune
dysfunction. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe
to this journal Receive 51 print issues and online access $199.00 per year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF
Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact
customer support SIMILAR CONTENT BEING VIEWED BY OTHERS COMBINATION ANTI-HIV ANTIBODIES PROVIDE SUSTAINED VIROLOGICAL SUPPRESSION Article 01 June 2022 PROLONGED VIRAL SUPPRESSION WITH
ANTI-HIV-1 ANTIBODY THERAPY Article Open access 13 April 2022 THERAPEUTIC EFFICACY OF COMBINED ACTIVE AND PASSIVE IMMUNIZATION IN ART-SUPPRESSED, SHIV-INFECTED RHESUS MACAQUES Article Open
access 16 June 2022 ACCESSION CODES ACCESSIONS GENBANK/EMBL/DDBJ * KF738375 * KF738446 DATA DEPOSITS The SHIV-AD8 gp120 sequences known to confer resistance to the 10-1074 or 3BNC117
monoclonal antibodies have been deposited in GenBank/EMBL/DDBJ under accession numbers KF738375 to KF738446. REFERENCES * Mascola, J. R. et al. Protection of macaques against pathogenic
simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. _J. Virol._ 73, 4009–4018 (1999) Article CAS Google Scholar * Moldt, B. et al. Highly potent
HIV-specific antibody neutralization _in vitro_ translates into effective protection against mucosal SHIV challenge _in vivo_. _Proc. Natl Acad. Sci. USA_ 109, 18921–18925 (2012) Article
ADS CAS Google Scholar * Nishimura, Y. et al. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus
challenge following passive transfer of high-titered neutralizing antibodies. _J. Virol._ 76, 2123–2130 (2002) Article CAS Google Scholar * Parren, P. W. et al. Antibody protects macaques
against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization _in vitro_. _J. Virol._ 75, 8340–8347 (2001) Article CAS
Google Scholar * Mehandru, S. et al. Adjunctive passive immunotherapy in human immunodeficiency virus type 1-infected individuals treated with antiviral therapy during acute and early
infection. _J. Virol._ 81, 11016–11031 (2007) Article CAS Google Scholar * Poignard, P. et al. Neutralizing antibodies have limited effects on the control of established HIV-1 infection
_in vivo_. _Immunity_ 10, 431–438 (1999) Article CAS Google Scholar * Trkola, A. et al. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human
neutralizing antibodies. _Nature Med._ 11, 615–622 (2005) Article CAS Google Scholar * Burton, D. R. et al. A blueprint for HIV vaccine discovery. _Cell Host Microbe_ 12, 396–407 (2012)
Article CAS Google Scholar * Huang, J. et al. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. _Nature_ 491, 406–412 (2012) Article ADS CAS Google Scholar *
Klein, F. et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. _Nature_ 492, 118–122 (2012) Article ADS CAS Google Scholar * Kong, L. et al.
Supersite of immune vulnerability on the glycosylated face of HIV-1 envelope glycoprotein gp120. _Nature Struct. Mol. Biol._ 20, 796–803 (2013) Article CAS Google Scholar * Walker, L. M.
et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. _Nature_ 477, 466–470 (2011) Article ADS CAS Google Scholar * Walker, L. M. et al. Broad and potent
neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. _Science_ 326, 285–289 (2009) Article ADS CAS Google Scholar * Wu, X. et al. Rational design of envelope
identifies broadly neutralizing human monoclonal antibodies to HIV-1. _Science_ 329, 856–861 (2010) Article ADS CAS Google Scholar * Gautam, R. et al. Pathogenicity and mucosal
transmissibility of the R5-tropic simian/human immunodeficiency virus SHIV(AD8) in rhesus macaques: implications for use in vaccine studies. _J. Virol._ 86, 8516–8526 (2012) Article CAS
Google Scholar * Nishimura, Y. et al. Generation of the pathogenic R5-tropic simian/human immunodeficiency virus SHIVAD8 by serial passaging in rhesus macaques. _J. Virol._ 84, 4769–4781
(2010) Article CAS Google Scholar * Shingai, M. et al. Most rhesus macaques infected with the CCR5-tropic SHIV(AD8) generate cross-reactive antibodies that neutralize multiple HIV-1
strains. _Proc. Natl Acad. Sci. USA_ 109, 19769–19774 (2012) Article ADS CAS Google Scholar * Walker, L. M. et al. Rapid development of glycan-specific, broad, and potent anti-HIV-1
gp120 neutralizing antibodies in an R5 SIV/HIV chimeric virus infected macaque. _Proc. Natl Acad. Sci. USA_ 108, 20125–20129 (2011) Article ADS CAS Google Scholar * Sadjadpour, R. et al.
Emergence of gp120 V3 variants confers neutralization resistance in an R5 simian-human immunodeficiency virus-infected macaque elite neutralizer that targets the N332 glycan of the human
immunodeficiency virus type 1 envelope glycoprotein. _J. Virol._ 87, 8798–8804 (2013) Article CAS Google Scholar * Mouquet, H. et al. Complex-type N-glycan recognition by potent broadly
neutralizing HIV antibodies. _Proc. Natl Acad. Sci. USA_ 109, E3268–E3277 (2012) Article CAS Google Scholar * Scheid, J. F. et al. Sequence and structural convergence of broad and potent
HIV antibodies that mimic CD4 binding. _Science_ 333, 1633–1637 (2011) Article ADS CAS Google Scholar * Zhou, T. et al. Structural basis for broad and potent neutralization of HIV-1 by
antibody VRC01. _Science_ 329, 811–817 (2010) Article ADS CAS Google Scholar * Diskin, R. et al. Restricting HIV-1 pathways for escape using rationally designed anti-HIV-1 antibodies.
_J. Exp. Med._ 210, 1235–1249 (2013) Article CAS Google Scholar * Horwitz, J. A. et al. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and
antiretroviral drugs in humanized mice. _Proc. Natl Acad. Sci. USA_ http://dx.doi.org/10.1073/pnas.1315295110 (2013) * Igarashi, T. et al. Human immunodeficiency virus type 1 neutralizing
antibodies accelerate clearance of cell-free virions from blood plasma. _Nature Med._ 5, 211–216 (1999) Article CAS Google Scholar * Keele, B. F. et al. Low-dose rectal inoculation of
rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. _J. Exp. Med._ 206, 1117–1134 (2009) Article CAS Google Scholar * Endo, Y. et al. Short- and
long-term clinical outcomes in rhesus monkeys inoculated with a highly pathogenic chimeric simian/human immunodeficiency virus. _J. Virol._ 74, 6935–6945 (2000) Article CAS Google Scholar
* Hansen, S. G. et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. _Nature_ 473, 523–527 (2011) Article ADS CAS Google Scholar * Montefiori,
D. C. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. _Curr. Protocols Immunol._ Ch. 12, Unit 12 11. (2005) * Polis, M. A. et al.
Correlation between reduction in plasma HIV-1 RNA concentration 1 week after start of antiretroviral treatment and longer-term efficacy. _Lancet_ 358, 1760–1765 (2001) Article CAS Google
Scholar Download references ACKNOWLEDGEMENTS We thank K. Tomioka and R. Kruthers for determining plasma viral RNA loads and B. Skopets, W. Magnanelli and R. Petros for diligently assisting
in the maintenance of animals and assisting with procedures. We also thank D. R. Burton, The Scripps Institute, for providing anti-dengue virus neutralizing monoclonal antibody (DEN-3). This
work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) and, in part, with federal funds from
the National Cancer Institute, NIH, under contract HHSN261200800001E. AUTHOR INFORMATION Author notes * Masashi Shingai and Yoshiaki Nishimura: These authors contributed equally to this
work. AUTHORS AND AFFILIATIONS * Laboratory of Molecular Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, 20892, Maryland, USA
Masashi Shingai, Yoshiaki Nishimura, Olivia K. Donau, Ronald Plishka, Alicia Buckler-White & Malcolm A. Martin * Laboratory of Molecular Immunology, The Rockefeller University, New York,
10065, New York, USA Florian Klein & Michel C. Nussenzweig * Department of Immunology, Laboratory of Humoral Response to Pathogens, Institut Pasteur, 75015 Paris, France, Hugo Mouquet *
Center for Virology and Vaccine Research, Beth Israel Deaconess Medical Center, Harvard Medical School, 3 Blackfan Circle, E/CLS-1001 Boston, 02115, Massachusetts, USA Michael Seaman * AIDS
and Cancer Virus Program, SAIC-Frederick, Inc., Frederick National Laboratory for Cancer Research, Frederick, Maryland 21702, USA, Michael Piatak & Jeffrey D. Lifson * Center for Cancer
Research, National Cancer Institute, National Institutes of Health, Bethesda, 20892, Maryland, USA Dimiter Dimitrov & Michel C. Nussenzweig * Howard Hughes Medical Institute, The
Rockefeller University, New York, 10065, New York, USA Michel C. Nussenzweig Authors * Masashi Shingai View author publications You can also search for this author inPubMed Google Scholar *
Yoshiaki Nishimura View author publications You can also search for this author inPubMed Google Scholar * Florian Klein View author publications You can also search for this author inPubMed
Google Scholar * Hugo Mouquet View author publications You can also search for this author inPubMed Google Scholar * Olivia K. Donau View author publications You can also search for this
author inPubMed Google Scholar * Ronald Plishka View author publications You can also search for this author inPubMed Google Scholar * Alicia Buckler-White View author publications You can
also search for this author inPubMed Google Scholar * Michael Seaman View author publications You can also search for this author inPubMed Google Scholar * Michael Piatak View author
publications You can also search for this author inPubMed Google Scholar * Jeffrey D. Lifson View author publications You can also search for this author inPubMed Google Scholar * Dimiter
Dimitrov View author publications You can also search for this author inPubMed Google Scholar * Michel C. Nussenzweig View author publications You can also search for this author inPubMed
Google Scholar * Malcolm A. Martin View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.S., Y.N., M.C.N. and M.A.M. designed the experiments;
M.S., Y.N., F.K., H.M., O.K.D., R.P., A.B.-W. and M.P. performed the experiments; M.S., Y.N., F.K., M.P., J.D.L., D.D., M.C.N. and M.A.M. analysed the data; and M.S., Y.N., M.C.N. and M.A.M.
wrote the manuscript. M.S. and Y.N. contributed equally to the work. CORRESPONDING AUTHORS Correspondence to Michel C. Nussenzweig or Malcolm A. Martin. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing financial interests. EXTENDED DATA FIGURES AND TABLES EXTENDED DATA FIGURE 1 TREATMENT OF SHIV-INFECTED MACAQUES WITH SINGLE ANTI-HIV-1
NEUTRALIZING MONOCLONAL ANTIBODIES. Plasma viral loads and total CD4+ T cell numbers before (the initial 84 days of the SHIV-AD8EO infection) and during single monoclonal antibody treatment
are shown. KZ6 and MB6 received the 3BNC117 monoclonal antibody and MB8 and MCN were administered the 10-1074 monoclonal antibody. Macaques MB7 and MD5 were not treated. EXTENDED DATA FIGURE
2 SGA ANALYSIS OF SELECTED SHIV-AD8EO GP120 SEQUENCES, PRESENT IN REBOUND VIRUS AFTER SINGLE MONOCLONAL ANTIBODY IMMUNOTHERAPY, AND KNOWN TO CONFER RESISTANCE TO 10-1074 OR 3BNC117
MONOCLONAL ANTIBODY. SGA was used to amplify plasma viral RNA after monoclonal antibody treatment from the plasma of animals KZ6 (day 28 (_n_ = 8)), MB6 (day 23 (_n_ = 8)), MB8 (day 23 (_n_
= 9)) and MCN (day 23 (_n_ = 8)). The gp120 sequences at the top are present in the SHIV-AD8EO molecular clone inoculum. Mutations conferring resistance are highlighted in red. EXTENDED DATA
FIGURE 3 CIRCULATING CD4+ T CELLS IN FIVE CHRONICALLY SHIV-INFECTED MACAQUES TREATED WITH TWO ANTI-HIV-1 NEUTRALIZING MONOCLONAL ANTIBODIES. Plasma viral loads and total CD4+ T-cell numbers
before (first 1,100 to 1,140 days) and during the first or second cycle of combination monoclonal antibody treatment are shown. EXTENDED DATA FIGURE 4 BAL CD4+ T CELLS IN FIVE CHRONICALLY
SHIV-INFECTED MACAQUES TREATED WITH TWO ANTI-HIV-1 NEUTRALIZING MONOCLONAL ANTIBODIES. Plasma viral loads and the percentage of CD4+ T cells in CD3+ gated BAL specimens, before (first 1,100
to 1,140 days) and during the first or second cycle of combination monoclonal antibody treatment, are shown. EXTENDED DATA FIGURE 5 SGA ANALYSIS OF SELECTED SHIV-AD8EO GP120 SEQUENCES KNOWN
TO CONFER RESISTANCE TO 10-1074 OR 3BNC117 MONOCLONAL ANTIBODY, BEFORE AND AFTER COMBINATION IMMUNOTHERAPY. A–E, Plasmas from animals DBZ3 (pre (_n_ = 8); day 49 post first treatment (_n_ =
10); day 24 post second treatment (_n_ = 8)) (A), DC99A (pre (_n_ = 10); day 57 post first treatment (_n_ = 6); day 41 post second treatment (_n_ = 7)) (B), DBXE (pre (_n_ = 9), day 28 (_n_
= 8)) (C), DCF1 (pre (_n_ = 14), day 28 (_n_ = 10)) (D) and DCM8 (pre (_n_ = 7), day 28 (_n_ = 11)) (E) were evaluated. The gp120 sequences at the top are present in the SHIV-AD8EO molecular
clone inoculum. Mutations conferring resistance are highlighted in red. EXTENDED DATA FIGURE 6 CD4+ T-CELL NUMBERS INCREASE DURING COMBINATION MONOCLONAL ANTIBODY TREATMENT OF
SHIV-AD8EO-INFECTED MACAQUES. A, B, Levels of viral RNA and total CD4+ T-cell/CD4+ T-cell subsets in symptomatic chronically infected macaques DBXE (A) and DCF1 (B). EXTENDED DATA FIGURE 7
ASSAYS TO IDENTIFY 10-1074- OR 3BNC117-SPECIFIC NEUTRALIZING ACTIVITIES IN THE PLASMA OF MONOCLONAL-ANTIBODY-TREATED MACAQUES. A, ID50 values measured in the TZM-bl neutralization assay of
10-1074 and 3BNC117 against HIV-1 strains that are sensitive to one but not the other broadly neutralizing antibody (that is, HIV-1 strain X2088_9 (10-1074 sensitive); HIV-1 strain Q769_d22
(3BNC117 sensitive)). B, Neutralizing activities in plasma before antibody administration (preP), but spiked with 0.01, 0.1, 1, 10 and 100 μg ml−1 of antibodies 10-1074 (blue) or 3BNC117
(green). Neutralizing activities are reported as plasma ID50 titres (left columns) and converted to antibody concentrations (right columns) based on measured ID50 values in A. EXTENDED DATA
FIGURE 8 MONOCLONAL ANTIBODY LEVELS IN THE PLASMAS OF MONOTHERAPY AND COMBINATION MONOCLONAL ANTIBODY MACAQUE RECIPIENTS. A–C, Macaques treated with one neutralizing monoclonal antibody (A);
macaques receiving two cycles of combination monoclonal antibody treatment (B); macaques receiving a single cycle of combination monoclonal antibody treatment (C). ID50 titres (left
columns) and monoclonal antibody concentrations (right columns) were measured in the indicated macaque plasma samples before (Prebleed) and after (Day) monoclonal antibody administration.
RELATED AUDIO NEWS & VIEWS AUTHOR LOUIS PICKER TALKS TO NATURE ABOUT TREATING HIV WITH SOME UNUSUALLY POTENT ANTIBODIES. POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE
FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Shingai, M., Nishimura, Y., Klein, F. _et al._ Antibody-mediated
immunotherapy of macaques chronically infected with SHIV suppresses viraemia. _Nature_ 503, 277–280 (2013). https://doi.org/10.1038/nature12746 Download citation * Received: 05 August 2013 *
Accepted: 11 October 2013 * Published: 30 October 2013 * Issue Date: 14 November 2013 * DOI: https://doi.org/10.1038/nature12746 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative